Key Points
CLL cells induce defects in T-cell LFA-1–mediated migration by altering Rho GTPase activation signaling, downregulating RhoA and Rac1, and upregulating Cdc42.
Lenalidomide repairs these T-cell defects by restoring normal Rho GTPase activation signaling.
Abstract
T lymphocytes have an essential role in adaptive immunity and rely on the activation of integrin lymphocyte function–associated antigen-1 (LFA-1) to mediate cell arrest and migration. In cancer, malignant cells modify the immune microenvironment to block effective host antitumor responses. We show for the first time that CD4 and CD8 T cells from patients with chronic lymphocytic leukemia (CLL) exhibit globally impaired LFA-1–mediated migration and that this defect is mediated by direct tumor cell contact. We show that following the coculture of previously healthy T cells with CLL cells, subsequent LFA-1 engagement leads to altered Rho GTPase activation signaling by downregulating RhoA and Rac1, while upregulating Cdc42. Of clinical relevance, repair of this T-cell defect was demonstrated using the immunomodulatory drug lenalidomide, which completely rescued adhesion and motility function by restoring normal Rho GTPase activation signaling. Our report identifies a novel cancer immune evasion mechanism whereby tumor cells induce Rho GTPase signaling defects in T cells that prevent appropriate LFA-1 activation and motility. We believe these findings identify important biomarkers and highlight the clinical utility of immunotherapy to rescue normal T-cell function in CLLs that are likely to have relevance in other cancers.
Introduction
Circulating CD4 and CD8 lymphocytes are critical for orchestrating immunological function. T-cell immune surveillance requires rapid adhesion and migration into lymph nodes or inflamed tissues, where they can engage and form immunological synapses with cognate antigen-presenting cells (APCs). The integrin lymphocyte function–associated antigen-1 (LFA-1) (CD11a/CD18; αLβ2) is a key regulator of these functions of T cells and, as a consequence, its activation must be tightly controlled.1,2 T-cell adhesion occurs on surfaces expressing CD54, the LFA-1 ligand, intercellular adhesion molecule-1 (ICAM-1), including high endothelial venules (HEVs) in the lymph nodes or postcapillary venules at sites of inflammation. LFA-1 is not constitutively active but instead has its activity regulated by signaling through other membrane receptors that are activated during an immune response, a process termed “inside-out signaling.” For example, inflammatory stimuli such as chemokine signaling activate LFA-1 from its bent, resting form to an extended active conformation, enabling the integrin to bind to the CD54 ligand.3 Adhesion to CD54 generates the external force required for stabilizing the high-affinity conformation and subsequent signaling back into the T cell.4 This is termed “outside-in signaling” and leads to the effector functions of adhesion and migration into the lymph node or injury site. Thus, LFA-1 can be thought of as a bidirectional signaling molecule controlling cytoskeleton-dependent T-cell activation.5-7
An emerging hallmark of cancer progression is the ability of the protumor inflammatory microenvironment to block effective immune surveillance in patients.8 There is now realization that the disappointing clinical activity of previous T-cell–targeted immunotherapies is likely contributed to by the inability of cancer patient T cells to overcome immunosuppressive mechanisms co-opted by tumor cells in the microenvironment.9 Thus, characterization of the immunosuppressive mechanisms active in cancer and identification of targeted treatment approaches will be required to repair immune function in cancer patients and to harness the full clinical potential of immunotherapy.
We have used chronic lymphocytic leukemia (CLL) as a model cancer to study T lymphocytes that are exposed to high numbers of constantly circulating tumor cells.10,11 We previously demonstrated that these T cells are dysfunctional compared with age-matched healthy donor T cells, and gene expression profiling studies revealed significant deregulation of multiple signaling pathway genes, including the Rho family GTPases and their regulators, the actin cytoskeleton and vesicle trafficking.12 This molecular analysis led to the characterization of impaired T-cell immune synapse function with APCs in CLL.13 We found that CD4 and CD8 T cells from CLL patients failed to form stable adhesive conjugates with APCs and had defective filamentous actin polymerization at the immune synapse. LFA-1 signaling at the T-cell synapse is required to form the peripheral supramolecular activation cluster that controls activation signaling.14 The CLL patient T cells showed reduced clustering of LFA-1 as well as reduced expression of high-affinity LFA-1 at the contact site with CD54-expressing APCs.13
In this present study, we investigated another major T-cell activity regulated by LFA-1 in T cells from CLL patients, namely, adhesion and migration on CD54. Our results show for the first time that leukemic cells induce a T-cell adhesion/migration defect that is mediated by dysregulated Rho GTPase signaling. Critically, we identified that the immunomodulatory drug lenalidomide restores normal Rac1, RhoA, and Cdc42 levels of activity in T cells from CLL patients and rescues LFA-1 function. Taken together, we believe that our results define defective T-cell migration as a novel immune evasion mechanism used by CLL cancer cells, and they identify a clinically relevant immunomodulatory agent for reversing this immune defect in cancer.
Materials and methods
Cell isolation and culture
All primary patient and age-matched healthy donor samples were obtained after written consent in accordance with the Declaration of Helsinki and were approved by the North London Research Ethics Committee (London, UK). All CLL patients (n = 94) were previously untreated (median time from diagnosis, 32 months [range 6-96]) at the time that heparinized venous blood samples were obtained for these studies. In vivo lenalidomide-derived samples came from a review board–approved clinical trial examining the efficiency of lenalidomide in previously treated symptomatic CLL patients.15,16 Patient and age-matched healthy donor mononuclear cells were separated by Ficoll-Hypaque density-gradient centrifugation. Healthy donor lymphocytes for the coculture assays were obtained from buffy coats prepared by the National Blood Service NHS Blood and Transplant (Brentwood, UK). CD3, CD4, and CD8 T cells were negatively selected using Miltenyi Biotec magnetic-activated cell sorting (MACS) cell isolation kits. Normal and malignant B cells were positively selected using MACS CD19 MicroBeads. An autoMACS Pro Separator was used for the cell sorting of viable, functionally active cells. The purity of isolated lymphocytes was always >95% (flow cytometry). Cell numbers and viability were measured using a Vi-CELL XR analyzer. Primary cells were maintained in RPMI 1640 medium containing 10% human serum for 24 hours before functional use.
Antibodies and reagents
Monoclonal antibodies (mAbs) used in this study were 38 (pan–LFA-1 specific for CD11a) and 24 (CD18 integrin activation reporter),1,17 all prepared at Cancer Research UK London Research Institute (London, UK). Five domain CD54-Fc was produced as previously described in Smith et al.18 Pooled AB human serum, penicillin-streptomycin, Alexa Fluor 488–labeled goat antimouse and Alexa Fluor 547–labeled goat antirabbit were all from Life Technologies. Phospho-mysoin light chain 2 (Ser19) Ab was from Cell Signaling Technology. Rap1 Ab was from BD Transduction Laboratories. Recombinant stromal cell–derived factor-1α (SDF-1α, CXCL12) and CCL19 used at 1 × 10−8 M were from PeproTech. Pharmacologic inhibitors for Rac1 (NSC-23766) used at 50 μM for 24 hours, the RhoA subfamily (C3 exoenzyme) used at 10 μM for 24 hours, and the ROCK (InSolution Rho kinase inhibitor) used at 10 μM for 15 minutes at 37°C were from Merck Millipore.19,20
Transfections
Primary T cells (5 × 106−1 × 107 cells) were washed into supplemented Nucleofector solution, according to Amaxa Nucleofector protocols for unstimulated human primary T cells, and nucleofected with the following reagents using settings optimized for transfection efficiency (V-024): 5μg maxGFP (Lonza), 5μg full-length constitutively active Cdc42 construct (V12) tagged with eGFP, or siRNA SMARTpool-targeted reagents (see supplemental Figure 3). Transfected cells were maintained in RPMI 1640 with 10% fetal calf serum for up to 24 hours before imaging.
Flow cytometry
We performed flow cytometry on an LSRFortessa cell analyzer (BD Biosciences) and analyzed data using FlowJo version 8.8.6 software (TreeStar). We gated live cells by forward and side scatter, DAPI (4,6 diamidino-2-phenylindole)–negative staining, and we ensured correct compensation and acquisition setup. Cells were resuspended at 1 × 106 in 100 μL of phosphate-buffered saline (PBS) containing 2% human serum. We incubated cells with pretitrated antibodies (Abs) to cell-surface markers at 4°C for 20 minutes. CXCR4 (PE) was from eBioscience. Results are expressed as median fluorescence intensity (MFI) corrected for nonspecific background staining (isotype control-PE; BD Biosciences).
Primary coculture screening assays
To analyze the impact on T cells of CLL direct contact, T cells (5 × 106/mL) were cocultured together (1:2 ratio) with tumor cells or healthy donor B cells (1 × 107/mL) in full culture medium for 48 hours in a 24-well culture plate. For cell adhesion blocking, T cells were cocultured with CLL cells that had been pretreated with blocking antibody (anti-CD54 10 μg/mL) for 1 hour and subsequently washed to remove any unbound antibody. All experiments were performed using isotype-matched IgG as controls. To analyze the impact on T cells of soluble-derived tumor factors only, T cells (lower well) were incubated with CLL cells or healthy allogeneic B cells (upper chamber) (1:2 ratio) in 24-well transwell plates (0.4-μm pore) for 48 hours. After coculture, cells were harvested by negative selection (using MACS CD20 and CD19 MicroBeads) for subsequent viability (Vi-CELL XR analyzer, >90%), flow cytometry purity analysis (>95%), and subsequent functional assays.
Video microscopy
Ibidi μ-Slides VI were coated with 3 μg/mL CD54-Fc in PBS (3 µg/mL) overnight at 4°C, then blocked with 2.5% bovine serum albumin (BSA) in PBS for 2 hours at room temperature and washed with Hanks balanced salt solution buffer (HBSS) containing 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (H.HBSS) at 4°C. T cells (4 × 105) were treated with SDF-1α in H.HBSS and allowed to settle for 10 minutes at 37°C. Nonattached cells were removed by gentle washing. Images were captured with a Nikon Diaphot 300 microscope, using an ×20 lens and AQM2001 Kinetic Acquisition Manager software (Kinetic Imaging). Cells were tracked at 15-second intervals with Motion Analysis software (Kinetic Imaging), and the data were analyzed using a Mathematica notebook (Wolfram Research) developed by D. Zicha (Cancer Research UK).21 The migration data are presented as box-and-whisker plots, where the line represents the median and the box and whiskers span 50% and 80% of the data, respectively.
Interference reflection microscopy (IRM)
T cells were plated on Ibidi μ-Slides VI coated with 3mg/mL CD54-Fc as described above. Images of the close substrate contact of migrating cells were obtained at both 10 and 20 minutes after initial attachment using a Zeiss LSM510 Axiovert 100 M inverted confocal microscope with a 63× NA1.4 Plan-Apochromat oil immersion objective lens.21 For evaluation of adhesion status (area of adhesion and pixel intensity), the area of contact within 20 nm of CD54 substrate was measured using MetaMorph Offline 7.1 software (n = 30 cells per experimental population).
Immunofluorescence labeling and confocal microscopy
Round, 13–mm, glass coverslips were precoated with CD54-Fc in PBS (3 µg/mL) overnight at 4°C, then blocked with 2.5% bovine serum albumin in PBS for 2 hours at room temperature and washed with H.HBSS at 4°C. Washed T cells were treated with SDF-1α and added to coated coverslips (2 × 105 cells per coverslip) for 30 minutes at 37°C. Adherent cells were fixed with 3% formaldehyde in PIPES buffer (pH 8) for 5 minutes at room temperature, washed in PBS, and fixed for a further 10 minutes in 3% formaldehyde in sodium tetraborate (Sigma-Aldrich) (pH 11). Cells were next permeabilized with 0.1% Triton-X-100 for 5 minutes on ice. Autofluorescence was quenched using 1 mg/mL sodium tetraborate (pH 8) for 15 minutes. Coverslips were incubated with primary monoclonal antibodies (mAbs) overnight at 4°C, followed by secondary Alexa Fluor Abs for 30 minutes. After washing, the cell specimens were then sealed with coverslips using fluorescent mounting medium (Dako). The specificity of staining was optimized and controlled using appropriate dilutions of isotype-control primary Abs and subsequent fluorescent secondary Abs. Medial optical section images were captured with a Zeiss 510 confocal laser-scanning microscope using a 63×/1.40 oil objective and LSM version 3.2 SP2 imaging software (Zeiss). Detectors were set to detect an optimal signal below saturation limits. Image sets to be compared were acquired during the same session using identical acquisition settings. Some images are depicted using a rainbow scale where fluorescence intensity is indicated by the difference in color.
Quantitative image analysis
Blinded confocal images were analyzed using AxioVision version 4.8 image analysis software (Zeiss).15 The AxioVision outline tool with densitometric mean analysis was then used to measure the total expression of high-affinity LFA-1 or phosphorylated myosin light chain (p-MLC) on each polarized T cell (n = 50 per experimental population). The AxioVision outline tool was also used to draw around each T cell (n = 50) plasma membrane (phase-contrast channel) or CD3+ plasma membrane to allow the extraction of the densitometric mean analysis of membrane Rap1 staining from the green fluorescence channel. These values were then normalized to total Rap1 staining values. These data were then exported into Prism version 5 software (GraphPad) for statistical analysis and to generate an MFI value per experimental population.
Rho GTPase activity assays
After primary coculture with primary CLL cells, healthy donor T cells (107cells at a 2:1 ratio) or T cells from CLL patients (107 cells untreated or pretreated with lenalidomide) were isolated by negative selection and resuspended in H.HBSS buffer. T cells were then incubated for 20 minutes at 37°C with 10 μg/mL pan–LFA-1 mAb (38) and SDF-1α. Cells were then washed in ice-cold PBS and then lysed (100 μL lysis buffer plus protease inhibitor cocktail), and RhoA, Rac1A, and Cdc42 activity was measured (490 nm colorimetric detection) according to the manufacturer’s protocols (BK124, BK128, and BK127 G-LISA kits; Cytoskeleton).
Lenalidomide treatment
Lenalidomide was a gift from Celgene Corporation. Drug powder was dissolved in dimethylsulfoxide (10 mM stock) and used to pretreat T cells at a final concentration of 1 μM in full culture medium (24 hours). Vehicle control–treated cells were cultured using dimethylsulfoxide alone.
Statistical methods
The 2-tailed unpaired or paired (measurements) Student t test was used to compare data between 2 experimental groups as appropriate using Prism version 5 software (GraphPad). P < .05 was considered significant.
Results
T cells from CLL patients exhibit impaired LFA-1–mediated motility that can be induced in previously healthy T cells via direct tumor cell contact
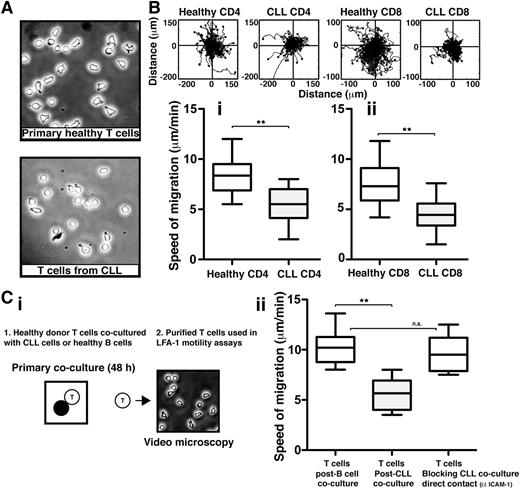
We first sought to assess the LFA-1–mediated cell migration of highly purified T cells from previously untreated CLL patients compared with those from age-matched healthy donors, using immobilized CD54 ligand and the chemokine CXCL12 to trigger LFA-1–mediated adhesion. Video microscopy was used to capture the random migration of these T-cell populations on CD54 before subsequent tracking and analysis. CLL patient T cells had a more rounded morphology compared with healthy T cells (Figure 1A). As expected, healthy CD4 and CD8 T cells characteristically moved rapidly with frequent changes in direction, indicating fully engaged migratory signaling machinery (n = 14 normal controls) (Figure 1B). In contrast, and in keeping with their less polarized morphology, both CD4 and CD8 T cells from CLL patients (n = 14) exhibited significantly reduced migration rates compared with healthy donor cells patients (P < .01) (Figure 1B and supplemental Videos 1 and 2, respectively).
T cells from CLL patients exhibit impaired LFA-1 – mediated motility that can be induced in healthy T cells via direct tumor cell contact. (A) Phase-contrast images (original magnification ×20) of primary T cells isolated from age-matched healthy donors (top) and CLL patients (bottom) were allowed to adhere to immobilized CD54 ligand for 10 minutes following exposure to chemokine CXCL12. (B) Video microscopy was then used to observe the migration of (Bi) CD4 or (Bii) CD8 T cells on CD54 for 20 minutes before the migration of individual cells was tracked. Representative migratory tracks of individual T cells from healthy donors compared with those of CLL patients are shown in the upper plots (n = 40 cells per patient experiment). Box-and-whisker plots of 14 independent CLL patient migration experiments are shown in the bottom box plots. (Ci) The image shows a schematic summary of 2-part coculture functional assays. Healthy donor CD3 T cells were cocultured (48 hours) with either CLL cells (untreated or pretreated with an anti-CD54 neutralizing mAb to block CLL/T cell contact) or third-party healthy B cells (control), and then purified for subsequent migration assay analysis on CD54. (Cii) Box-and-whisker plot analysis of 14 independent CLL patient migration experiments is shown. **P < .01. ns, nonsignificant findings.
T cells from CLL patients exhibit impaired LFA-1 – mediated motility that can be induced in healthy T cells via direct tumor cell contact. (A) Phase-contrast images (original magnification ×20) of primary T cells isolated from age-matched healthy donors (top) and CLL patients (bottom) were allowed to adhere to immobilized CD54 ligand for 10 minutes following exposure to chemokine CXCL12. (B) Video microscopy was then used to observe the migration of (Bi) CD4 or (Bii) CD8 T cells on CD54 for 20 minutes before the migration of individual cells was tracked. Representative migratory tracks of individual T cells from healthy donors compared with those of CLL patients are shown in the upper plots (n = 40 cells per patient experiment). Box-and-whisker plots of 14 independent CLL patient migration experiments are shown in the bottom box plots. (Ci) The image shows a schematic summary of 2-part coculture functional assays. Healthy donor CD3 T cells were cocultured (48 hours) with either CLL cells (untreated or pretreated with an anti-CD54 neutralizing mAb to block CLL/T cell contact) or third-party healthy B cells (control), and then purified for subsequent migration assay analysis on CD54. (Cii) Box-and-whisker plot analysis of 14 independent CLL patient migration experiments is shown. **P < .01. ns, nonsignificant findings.
CLL patient samples were selected to represent the heterogeneity of the disease, including different stages of disease, IgVH mutational status, and ZAP-70 status. Dysfunctional T-cell migration was observed consistently across the disease spectrum.10,22,23 T cells from CLL patients exhibited similar reduced migration rates on CD54 following stimulation with CCL19, and there was no difference in the expression of chemokine receptor CCR7 or CXCR4 detected between T cells from CLL patients and healthy donor T cells (supplemental Figure 1A-C).24
We have previously demonstrated that direct contact interaction with CLL tumor cells suppresses the ability of healthy T cells to form immunological synapses effectively with APCs.13 We therefore investigated if interaction with tumor cells also drives the T-cell LFA-1 motility defect. To test this, we used a 2-part coculture assay (Figure 1Ci). First, healthy donor T cells were cocultured for 48 hours with either allogeneic CLL cells or third-party healthy donor B cells acting as control cells. Second, these T cells were purified by negative selection and used in subsequent migration assays on purified immobilized CD54 ligand. Primary coculture with CLL cells caused a significant decrease in the speed of T-cell migration compared with coculture with healthy B cells (P < .01) (Figure 1Cii). Notably, pretreatment of CLL cells with an anti-CD54 neutralizing mAb to block intercellular contact of tumor cells with T cells in primary coculture prevented subsequent induction of the migration defect. In addition, primary transwell culture assays showed that tumor soluble factors alone did not induce identifiable LFA-1 motility defects in T cells (supplemental Figure 2). Together, these studies identify reduced LFA-1 motility as a major functional defect in T cells from CLL patients that is induced by direct contact with tumor cells.
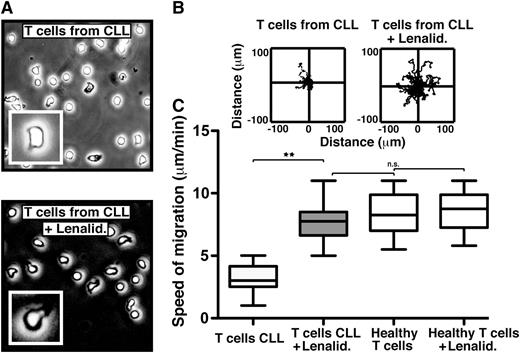
The immunomodulatory drug lenalidomide repairs the LFA-1–mediated motility defect in CLL patient T cells via cereblon
In order to investigate a clinically relevant approach to improving T-cell LFA-1 motility in cancer patients, we analyzed the effect of the immunomodulatory drug lenalidomide, which is clinically active in CLL and other hematologic cancers.25,26 The exact mechanisms of action of this drug are not fully defined, but they include activation of antitumor immunity in the immune microenvironment.27 We have previously shown that lenalidomide repairs the ability of T cells from CLL patients to form functional immune synapses with APCs.13 Treatment of cancer patient T cells with lenalidomide completely restored rapid LFA-1–mediated migration on CD54 to speeds comparable to healthy donor T cells (Figure 2 and supplemental Video 3). Cereblon expression is required for lenalidomide-mediated induced T-cell activation (interleukin IL-2 production).28 Knockdown (siRNA) of cereblon in CLL patient T cells also significantly reduced the ability of lenalidomide to modulate migration on CD54 (supplemental Figure 3), providing further evidence that cereblon is a direct proximate molecular target for the immunomodulatory activity of lenalidomide. Interestingly, lenalidomide did not modulate healthy T-cell motility speeds, possibly indicating a saturated motility function that is insensitive to drug stimulation and that lenalidomide repairs a defect within the T cells of the cancer-bearing patients. Direct clinical relevance was confirmed by the analysis of the migration of T cells obtained during an in vivo lenalidomide clinical trial in CLL.15,16 The T cells from the drug-treated patients showed significantly increased LFA-1–mediated migration compared with baseline untreated samples (P < .01) (supplemental Figure 4). To our knowledge, these data demonstrate for the first time the repair of defective T-cell LFA-1–mediated migration in cancer using an immunomodulatory drug.
The immunomodulatory drug lenalidomide repairs the LFA-1 – mediated motility defect in CLL patient T cells. (A) Phase-contrast images (original magnification ×20) show untreated (top) and lenalidomide (Lenalid.) (1μM)–treated (bottom) CD3 T cells from CLL patients, which were adhering to immobilized CD54 ligand for 10 minutes following exposure to chemokine CXCL12. The enlarged image (inset) shows an individual cell from a representative experiment. Video microscopy recorded the migration on CD54 for 20 minutes before tracking and analysis. Representative migratory tracks of individual T cells from untreated compared with Lenalid.-treated CLL samples are shown in (B) the upper plots (n = 40 cells per patient experiment). (C) Box-and-whisker plot analysis of 12 independent CLL patient migration experiments is shown in the bottom box plots. **P < .01. ns, nonsignificant findings.
The immunomodulatory drug lenalidomide repairs the LFA-1 – mediated motility defect in CLL patient T cells. (A) Phase-contrast images (original magnification ×20) show untreated (top) and lenalidomide (Lenalid.) (1μM)–treated (bottom) CD3 T cells from CLL patients, which were adhering to immobilized CD54 ligand for 10 minutes following exposure to chemokine CXCL12. The enlarged image (inset) shows an individual cell from a representative experiment. Video microscopy recorded the migration on CD54 for 20 minutes before tracking and analysis. Representative migratory tracks of individual T cells from untreated compared with Lenalid.-treated CLL samples are shown in (B) the upper plots (n = 40 cells per patient experiment). (C) Box-and-whisker plot analysis of 12 independent CLL patient migration experiments is shown in the bottom box plots. **P < .01. ns, nonsignificant findings.
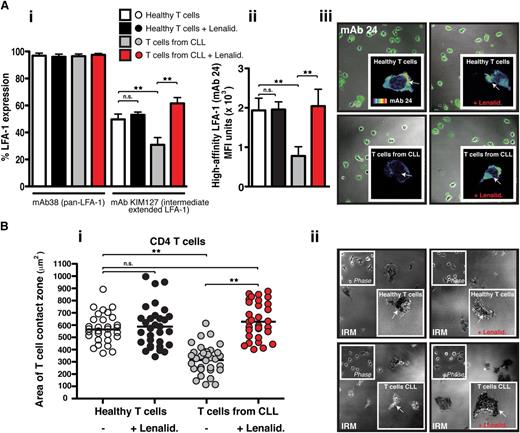
Lenalidomide increases both intermediate- and high-affinity LFA-1 expression on CLL-derived T cells, rescuing their firm adhesion to CD54
Activation of LFA-1 on T cells is essential for firm adhesion to and migration on its ligand CD54.29 Chemokine stimulation leads to the induction of inside-out signaling and the extension of the bent low-affinity integrin conformation to an extended conformation where LFA-1 can assume either intermediate- or high-affinity conformations for CD54. To measure activation of LFA-1, we used mAbs that recognize conformation-sensitive epitopes, including mAbs that recognize intermediate- (KIM127) and high-affinity (24) epitopes.1 Flow cytometric analysis of T cells in suspension using a pan–LFA-1 mAb showed that there was no difference in total LFA-1 integrin expression in CLL patients and age-matched healthy donor T cells (Figure 3Ai). However, T cells from CLL patients expressed significantly less chemokine-triggered extended intermediate-affinity LFA-1 compared with age-matched healthy donor T cells (P < .01). Treatment of these patients’ T cells with lenalidomide significantly increased the transition to the extended active conformation (P < .01). In contrast, no effect was detected on healthy T cells. Of note, primary coculture of previously healthy donor T cells with CLL cells induced a similar reduction in intermediate-affinity LFA-1 expression compared with coculture with healthy B cells (P < .01) (supplemental Figure 5).
Lenalidomide increases extended intermediate- and high-affinity LFA-1 expression on CLL-derived T cells and rescues their firm adhesion to CD54. (Ai) The graph shows the percent of positive flow cytometric expression analysis of pan–LFA-1 (mAb 38) and the extended intermediate-affinity LFA-1 (KIM127 mAb) on chemokine CXCL12–treated suspension cells (columns show the mean ± SEM from 6 patients). (Aii) The graph shows MFI ± SD of high-affinity LFA-1 (mAb 24) immunofluorescent staining during T-cell migration on CD54 (3 independent CLL patient experiments) examining untreated or lenalidomide (Lenalid.)–treated peripheral blood CD3 T cells from CLL patients compared with age-matched healthy donor cells. (Aiii) Representative images of mAb 24 staining visualized using confocal microscopy (with Alexa Fluor 488 secondary antibody) are shown for each experimental T-cell population as indicated. The enlarged images (insets) show single-cell mAb 24 staining using a rainbow scale where yellow/red indicates strong expression and blue the weakest). (Bi) The graph shows IRM quantification of the area of close cell contact points during LFA-1–directed motility on CD54 (following chemokine stimulation) for n = 30 T cells (individual dots) per experimental population (dot plots are representative of 3 independent CLL patient experiments). (Bii) Representative cell contact points are shown in the IRM images of T cells from healthy donors (upper panels) compared with CLL patients (lower panels) who were untreated (left panels) and treated with lenalidomide (Lenalid.) (right panels). Phase-contrast images are shown (upper left insets) as are enlarged single-cell IRM images (lower right insets). Original magnification ×63.**P < .01. ns, nonsignificant findings.
Lenalidomide increases extended intermediate- and high-affinity LFA-1 expression on CLL-derived T cells and rescues their firm adhesion to CD54. (Ai) The graph shows the percent of positive flow cytometric expression analysis of pan–LFA-1 (mAb 38) and the extended intermediate-affinity LFA-1 (KIM127 mAb) on chemokine CXCL12–treated suspension cells (columns show the mean ± SEM from 6 patients). (Aii) The graph shows MFI ± SD of high-affinity LFA-1 (mAb 24) immunofluorescent staining during T-cell migration on CD54 (3 independent CLL patient experiments) examining untreated or lenalidomide (Lenalid.)–treated peripheral blood CD3 T cells from CLL patients compared with age-matched healthy donor cells. (Aiii) Representative images of mAb 24 staining visualized using confocal microscopy (with Alexa Fluor 488 secondary antibody) are shown for each experimental T-cell population as indicated. The enlarged images (insets) show single-cell mAb 24 staining using a rainbow scale where yellow/red indicates strong expression and blue the weakest). (Bi) The graph shows IRM quantification of the area of close cell contact points during LFA-1–directed motility on CD54 (following chemokine stimulation) for n = 30 T cells (individual dots) per experimental population (dot plots are representative of 3 independent CLL patient experiments). (Bii) Representative cell contact points are shown in the IRM images of T cells from healthy donors (upper panels) compared with CLL patients (lower panels) who were untreated (left panels) and treated with lenalidomide (Lenalid.) (right panels). Phase-contrast images are shown (upper left insets) as are enlarged single-cell IRM images (lower right insets). Original magnification ×63.**P < .01. ns, nonsignificant findings.
Next we used confocal microscopy with mAb 24 to measure high-affinity LFA-1 expression on T cells polarized on CD54. T cells from CLL patients had significantly less high-affinity LFA-1 expression compared with age-matched healthy donor cells (P < .01) (Figure 3Aii-iii). However, pretreatment of these patients’ T cells with lenalidomide significantly increased high-affinity LFA-1 expression (P < .01), whereas no effect was detected on healthy T cells. To explore further the expression of high-affinity LFA-1, we used interference reflection microscopy (IRM) to measure T-cell adhesion to CD54. This imaging approach permits visualization of regions of dark contrast representing cell contact points within 20 nm of the CD54 surface. These adhesive contacts can be quantified in terms of size (proportional to area) and strength (proportional to pixel intensity). Both CD4 and CD8 T cells from healthy donors exhibited strong adhesion on CD54 that was unaffected by lenalidomide treatment (Figure 3Bi-ii and supplemental Figure 6). In contrast, T cells from CLL patients had significantly reduced areas (Figure 3Bi and supplemental Figure 6A) and strength (supplemental Figure 6B and C) of cell membrane in contact with CD54 (P < .01). However, pretreatment of these patients’ T cells with lenalidomide significantly increased both the size and the strength of contacts made by the LFA-1–expressing membrane on CD54 (P < .01), allowing a complete rescue of normal cell adhesion. Additional control IRM experiments were performed treating T cells from CLL patients with manganese (Mn2+) buffer (without chemokine stimulation) that bypasses inside-out signaling and directly activates LFA-1 (supplemental Figure 7). These experiments showed that Mn2+ buffer–treated T cells showed significantly increased strength of cell adhesion on CD54 compared with untreated CLL patient T cells. Taken together, these results demonstrate that lenalidomide targets regulatory pathways controlling the extension and activation of LFA-1 on CLL T cells. Furthermore, LFA-1 has the potential to be functional, but contact with tumor cells induces defective inside-out signaling, preventing normal LFA-1 affinity triggering.
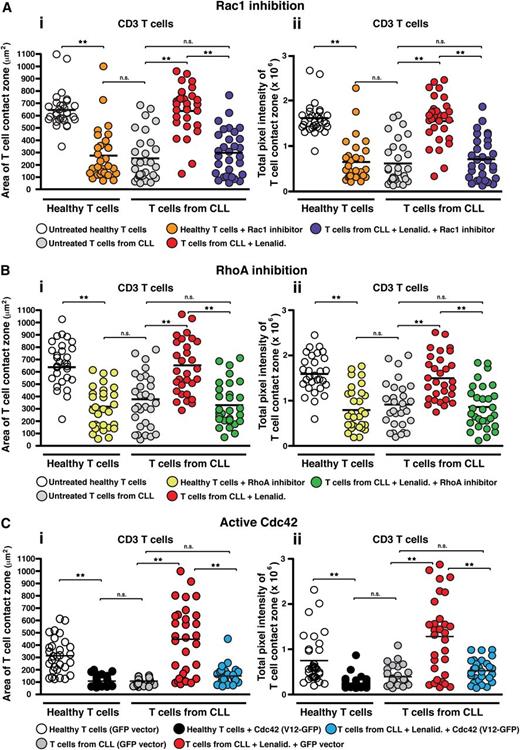
Lenalidomide targets Rho GTPase signaling in T cells from CLL patients
As our previous gene expression profiling analysis of T cells from CLL patients revealed abnormal gene expression of Rho GTPases including upregulated Cdc42,12 we next focused our attention on these kinases to investigate whether they might be involved in the dysregulated LFA-1 activity. Rho GTPases cycle between an active guanosine triphosphate–bound form and an inactive guanosine diphosphate–bound form and are key signal transducer molecules shown to regulate T-cell activity, including migration.30,31 Our approach was to combine the use of pharmacologic inhibitors selective for RhoA and Rac or transfection with a constitutive active mutant for Cdc42 to target the activity of these signaling molecules, with IRM imaging of T-cell adhesion during LFA-1–directed motility on CD54 as the readout. Pretreatment of healthy donor T cells with an inhibitor of Rac1 (Figure 4Ai-ii), the RhoA subfamily (Figure 4Bi-ii), the RhoA effector protein Rho kinase (ROCK) (supplemental Figure 8i-ii), or constitutive active Cdc42 (V12-GFP construct) (Figure 4Ci-ii) induced a CLL T-cell phenotype with reduced area of cell spreading and strength as measured by pixel intensity. We confirmed these findings by siRNA knockdown of these Rho GTPases (data not shown). Treatment of CLL patient T cells with lenalidomide significantly increased LFA-1–mediated adhesive activity (P < .01), but this was blocked following cotreatment of lenalidomide with the Rho GTPase inhibitors or with constitutively active Cdc42. Of note, the inhibitor-induced CLL T-cell phenotype in healthy donor cells was prevented with lenalidomide cotreatment (data not shown). Taken together, these results suggest that impaired LFA-1–mediated adhesion of CLL T cells is associated with dysregulated RhoA, Rac, and Cdc42 GTPase signaling pathways and that this aberrant GTPase activity is reversible with lenalidomide.
Lenalidomide targets Rho GTPase signaling in T cells from CLL patients. IRM quantification of the (Ai) area (μm2) and (Aii) strength (pixel intensity) of close cell contact points during LFA-1–directed motility on CD54 (following chemokine CXCL12 stimulation) for n = 30 T cells (individual dots) per experimental population is depicted, as indicated. T cells from CLL patients were compared with age-matched healthy donors treated with or without a pharmacologic inhibitor targeting (A) Rac1 or (B) the RhoA subfamily, or (C) transfected with GFP vector alone or with constitutive active Cdc42 (GFP-tagged V12 construct) with cells selected for analysis on the basis of GFP expression. Patient cells were analyzed with or without cotreatment of lenalidomide (Lenalid.). Dot plots are representative of 3 independent CLL patient experiments. **P < .01. black horizontal bar, mean values; ns, nonsignificant findings.
Lenalidomide targets Rho GTPase signaling in T cells from CLL patients. IRM quantification of the (Ai) area (μm2) and (Aii) strength (pixel intensity) of close cell contact points during LFA-1–directed motility on CD54 (following chemokine CXCL12 stimulation) for n = 30 T cells (individual dots) per experimental population is depicted, as indicated. T cells from CLL patients were compared with age-matched healthy donors treated with or without a pharmacologic inhibitor targeting (A) Rac1 or (B) the RhoA subfamily, or (C) transfected with GFP vector alone or with constitutive active Cdc42 (GFP-tagged V12 construct) with cells selected for analysis on the basis of GFP expression. Patient cells were analyzed with or without cotreatment of lenalidomide (Lenalid.). Dot plots are representative of 3 independent CLL patient experiments. **P < .01. black horizontal bar, mean values; ns, nonsignificant findings.
CLL tumor cells induce altered LFA-1–mediated Rho GTPase activity
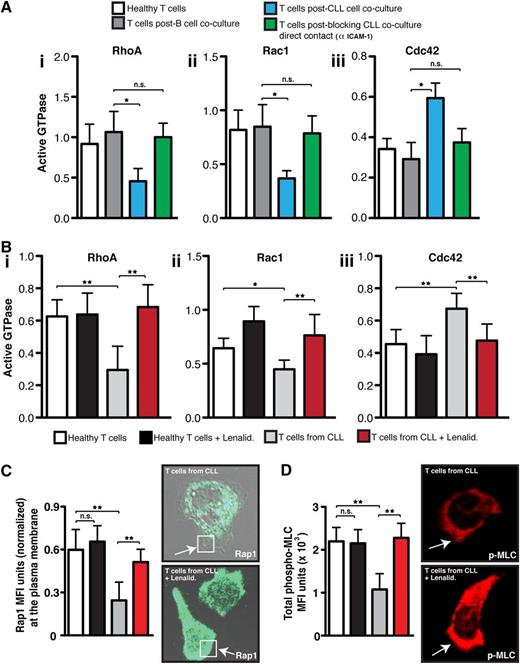
Rho GTPases exert their signaling activity by converting to an active guanosine triphosphate–bound conformation that allows them to bind readily to effector proteins that mediate downstream signaling pathways.30 We next wanted to test directly whether the ability of CLL cells to suppress adhesion and migration of healthy donor T cells occurred by interference with small GTPase activation. Primary coculture of control T cells with CLL cells, compared with age-matched healthy B cells, significantly decreased activated RhoA and Rac1 but increased Cdc42 levels (P < .05) in T cells following chemokine stimulation and LFA-1 cross-linking (Figure 5Ai-iii). This is in agreement with our previous data showing dysregulation of total Rho GTPase RNA and protein expression in tumor-exposed T cells compared with control cells.12,13 mAb blockade of CD54 expressed on CLL cells to prevent tumor cell/T-cell contact in primary coculture prevented the induction of these Rho GTPase signaling defects.
CLL tumor cells induce altered LFA-1 – mediated Rho GTPase activity. (A) The graphs show healthy donor CD3 T cells that were cocultured (48 hours) with either CLL cells (untreated or pretreated with an anti-CD54 neutralizing mAb to block CLL/T cell contact) or third-party healthy B cells (control). T cells from primary coculture or from (B) untreated or lenalidomide (Lenalid.)–treated CLL patients were then purified before exposure to CXCL12 chemokine and LFA-1 cross-linking (mAb 38). T cells were then lysed, and (Bi) RhoA, (Bii) Rac1, and (Biii) Cdc42 activity were measured using G-LISA assays (absorbance at 490 nm). (C) The colored columns show the combined mean activation signal ± SD from 6 CLL patients or healthy donor T cell controls (mean fluorescence expression ± SD). Normalized MFI of Rap1 immunofluorescent staining at the T-cell plasma membrane (C, white box) and (D) total MFI expression of p-MLC from 3 independent experiments (mean ± SD) examining untreated or lenalidomide-treated T cells from CLL patients compared with age-matched healthy donors cells migrating on immobilized CD54 are shown. Representative images of Rap1 and p-MLC staining visualized using confocal microscopy (with Alexa Fluor 488 or 546 secondary antibodies, respectively) for untreated and lenalidomide-treated T cells from CLL patients are shown as indicated. Original magnification ×63.*P < .05; **P < .01. ns, nonsignificant findings.
CLL tumor cells induce altered LFA-1 – mediated Rho GTPase activity. (A) The graphs show healthy donor CD3 T cells that were cocultured (48 hours) with either CLL cells (untreated or pretreated with an anti-CD54 neutralizing mAb to block CLL/T cell contact) or third-party healthy B cells (control). T cells from primary coculture or from (B) untreated or lenalidomide (Lenalid.)–treated CLL patients were then purified before exposure to CXCL12 chemokine and LFA-1 cross-linking (mAb 38). T cells were then lysed, and (Bi) RhoA, (Bii) Rac1, and (Biii) Cdc42 activity were measured using G-LISA assays (absorbance at 490 nm). (C) The colored columns show the combined mean activation signal ± SD from 6 CLL patients or healthy donor T cell controls (mean fluorescence expression ± SD). Normalized MFI of Rap1 immunofluorescent staining at the T-cell plasma membrane (C, white box) and (D) total MFI expression of p-MLC from 3 independent experiments (mean ± SD) examining untreated or lenalidomide-treated T cells from CLL patients compared with age-matched healthy donors cells migrating on immobilized CD54 are shown. Representative images of Rap1 and p-MLC staining visualized using confocal microscopy (with Alexa Fluor 488 or 546 secondary antibodies, respectively) for untreated and lenalidomide-treated T cells from CLL patients are shown as indicated. Original magnification ×63.*P < .05; **P < .01. ns, nonsignificant findings.
To validate these findings, we measured Rho GTPase activation expression profiles in T cells from CLL patients compared with age-matched healthy control cells following LFA-1 engagement. T cells from CLL patients exhibited similar dysregulated activation signaling to tumor-exposed T cells (Figure 5Bi-iii). Of note, lenalidomide treatment of T cells from CLL patients restored Rho GTPase signaling to healthy control cell levels. In further support of this reversible activation-signaling defect, treatment of CLL patient T cells with lenalidomide rescued small GTPase Rap1 trafficking to the membrane of motile lymphocytes, which is a critical step of high-affinity LFA-1 activation and cytoskeleton signaling (Figure 5C and supplemental Figure 9).4 Moreover, expression of the phosphorylated Rho GTPase effector protein myosin light chain that is an important downstream signaling molecule during LFA-1–mediated T-cell motility was rescued with drug treatment (Figure 5D).1
Discussion
In this study, we investigated the ability of T cells from CLL patients to undergo LFA-1–mediated migration. T cells rely on this promigratory integrin to leave the blood circulation and migrate into lymph nodes, where they scan for antigen-laden APCs. Time-lapse video microscopy with quantitative image analysis revealed that both CD4 and CD8 T cells from CLL patients show reduced speeds of migration compared with age-matched healthy control T cells. This functional defect was detected in all CLL patient samples tested irrespective of the underlying genetic abnormalities associated with this leukemia.10,22 We further demonstrated that direct contact coculture of CLL cells with previously healthy donor T cells induced this LFA-1 motility dysfunction. Blocking CLL/T-cell direct contact, by pretreating CLL cells with neutralizing anti-CD54 antibody, prevented induction of the defect. Moreover, soluble factors alone did not alter the motility speeds of healthy donor T cells. Taken together, these data show that the CLL tumor B cells have a direct role in mediating dysfunctional LFA-1–directed T-cell migration. To our knowledge, this is the first definition of a global T-cell migration defect in a cancer, a finding that has relevance for both disease biology and immunotherapy.
Translational clinical relevance was demonstrated by the rescue of the T-cell defects with lenalidomide, a drug that has shown considerable promise as both a single and combinational agent in CLL.25,32 Its mechanism of action needs further definition, but the evidence to date points to immunomodulatory activity rather than a direct pro-apoptotic effect in CLL,27 mediated via cereblon binding.28 We have previously demonstrated that lenalidomide treatment repairs T-cell synapse signaling defects in CLL.13 Here we show that both in vitro and in vivo lenalidomide treatment restores the expression of LFA-1 intermediate- and high-affinity conformations and LFA-1–directed migration to that of healthy T cells. Firm adhesion mediated by high-affinity LFA-1 is required at the T-cell midcell lamellar focal zone region during LFA-1–driven migration.1 These results suggest that an aspect of the switch from the bent, inactive conformation to the extended, active form of LFA-1 is impaired in T cells from CLL patients.
Our results pinpoint a GTPase module consisting of Rac1, RhoA/ROCK, and Cdc42 to be key targets of lenalidomide activity, as treatment with inhibitors selective for these kinases blocked the ability of the drug to rescue T-cell adhesion and motility. The small GTPases RhoA, Rac1, and Cdc42 are involved in the chemokine-induced triggering of the LFA-1 affinity state. RhoA and Rac1 are positive regulators of LFA-1 high affinity, whereas, conversely, Cdc42 inhibits the activation of high-affinity LFA-1.33,34 Our findings indicate that downregulated RhoA and Rac1 and potentiated Cdc42 activity in CLL T cells represent an important signaling block promoting negative regulation of LFA-1 activity.
Rho family GTPases and their regulators have important roles in adhesion and migration in keeping with their effects on LFA-1 activity and presumably also on the cytoskeleton.30,31 We showed that primary coculture with CLL cells downregulates active RhoA and Rac1 and increased Cdc42 expression in previously healthy T cells following chemokine and LFA-1 engagement compared with coculture with age-matched healthy B cells. Moreover, autologous T cells from CLL patients exhibit the same dysregulated Rho GTPase activation following integrin engagement. Motility requires constant dynamic reorganization of the cytoskeleton, and the activity of the Rho family GTPases is implicated in this turnover in T cells when they are migrating on CD54.30 RhoA regulates actomyosin contractility at the rear uropod required for LFA-1 de-adhesion, and also for protrusion and retraction at the leading edge.20,35 Rac1 also has a role in actin polymerization and LFA-1–mediated spreading at the leading edge.36 Cdc42 is associated with the control of T-cell polarization but may also contribute to actin polymerization at the leading edge and formation of filopodia during migration on CD54.31 Forced Cdc42 activity is associated with a general loss of lamellipodium and T-cell polarity.37 Myosin forms a signaling module with RhoA/ROCK to regulate rear uropod contraction during migration.20,31,38 We detected reduced expression of its p-MLC, an event downstream of Rho/ROCK, in T cells from CLL patients during migration on CD54. Lenalidomide treatment rescued all these functions to levels found in normal T cells.
These findings are in agreement with the differentially expressed GTPase and integrin regulatory genes in T cells from CLL patients compared with age-matched healthy donors.12 Gene and protein expression changes included upregulated GTPase-activating proteins (which negatively regulate GTPase activity) and Cdc42, while they downregulated RhoA and Rac1 guanine-exchange factors (GEFs, which positively regulate GTPase activity) such as Vav1 and Dock2 and the Rap1 GEF RasGRP2 (calcium and diacyglycerol-regulated guanine exchange factor 1, CalDAG-GEF1).12,39
Although it is not known which signaling receptors on CLL cells trigger the altered behavior in T cells, we have previously identified that the aberrant activity of B7 family members induces defective immune synapse signaling,15 and we are currently investigating whether these same immunosuppressive signals are involved in dysregulated integrin signaling. However, the mechanisms that control the conformational changes giving rise to the extended form of LFA-1 are becoming better understood.4 The location of the GTPase Rap1 at the membrane is a key element in LFA-1–activation priming and signaling. Reduced trafficking of this key inside-out signaling kinase to the LFA-1–expressing membrane and the reduced gene expression of its GEFs including RasGRP2 in CLL T cells strongly support this attenuated LFA-1 activating signaling model.40 A signaling defect in the Rap1 activation pathway would then set the stage for the subsequent impaired LFA-1–mediated adhesion and migration on CD54.
There are several possible links between Rho family GTPase signaling and high-affinity LFA-1 in T cells that controls migration. Agonist-induced inside-out signaling leads to Rap1 activity and extension of the bent-to-extended conformation of LFA-1. Subsequent events such as T-cell ZAP-70 activation, which are part of outside-in signaling occurring after contact with CD54, can lead directly to expression of high-affinity LFA-1.41,42 There is now good evidence that through the allosteric effects promoted by the stiffening effect of shear force on the β subunit, LFA-1 linked into the actin cytoskeleton is rapidly converted to high-affinity LFA-1 conformation.4,41,43 In this way, the Rho family GTPases provide a link between LFA-1 and the actin cytoskeleton. Whether there is a direct link between Rap1 activity and the Rho GTPase family is currently unknown.
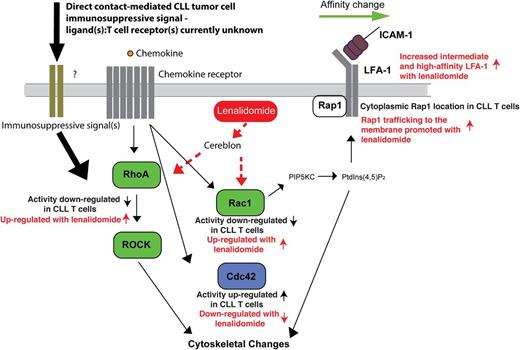
Taken together, the identification of tumor-induced T-cell Rho GTPase signaling defects points to a model of suppressed LFA-1 activation signaling and migration (Figure 6). Under physiological conditions, this switch is controlled by linkage to the cytoskeleton controlled by the Rho GTPases. Dysregulated Rho GTPase activity would suppress subsequent outside-in signaling and LFA-1–mediated adhesion and migration. The ability of lenalidomide to restore normal T-cell Rho GTPase activity and LFA-1 activation provides, for the first time, an important molecular definition of its mechanism of action, representing a powerful immunotherapeutic approach to rescue global T-cell motility defects in cancer patients. This study thus contributes to an understanding of the signaling pathways that drive abnormal integrin activation and function in T cells from CLL patients, and it will contribute to the design of immunotherapeutic strategies that promote effective tumor immune surveillance.
Schematic representation of CLL-induced Rho GTPase signaling defects in T cells that suppress LFA-1 activation signaling and migration and how this is reversible with lenalidomide. Direct contact of CLL cells with T cells (immunosuppressive signaling) downregulates RhoA and Rac1 (positive regulators of LFA-1 activation, green) and potentiates Cdc42 activity (negatively regulates LFA-1 activation, blue) in T cells inducing an important signaling block that negatively regulates LFA-1 activity. This attenuated LFA-1 activating signaling model is supported by reduced expression and trafficking of Rap1 at the LFA-1–expressing membrane in CLL T cells. PIP5KC (phosphatidylinositol-4-phosphate 5-kinase type I γ) and PtdIns(4,5)P2 (phosphatidylinositol-4,5-bisphosphate) are critical for LFA-1 affinity modulation downstream of RhoA and Rac. Diminished Rho GTPase activity and cytoskeletal signaling would suppress subsequent outside-in signaling and LFA-1–mediated adhesion and migration on the CD54 ligand. The ability of lenalidomide (red) to restore normal T-cell Rho GTPase activity and LFA-1 activation provides, for the first time, an important molecular definition of its mechanism of action. Cereblon expression is required for this immunomodulatory activity of lenalidomide. Dotted lines indicate that it is yet to be determined if lenalidomide activates Rho GTPases directly or indirectly via the cereblon pathway. These results indicate that lenalidomide represents a powerful immunotherapy to rescue global T-cell motility defects in cancer patients.
Schematic representation of CLL-induced Rho GTPase signaling defects in T cells that suppress LFA-1 activation signaling and migration and how this is reversible with lenalidomide. Direct contact of CLL cells with T cells (immunosuppressive signaling) downregulates RhoA and Rac1 (positive regulators of LFA-1 activation, green) and potentiates Cdc42 activity (negatively regulates LFA-1 activation, blue) in T cells inducing an important signaling block that negatively regulates LFA-1 activity. This attenuated LFA-1 activating signaling model is supported by reduced expression and trafficking of Rap1 at the LFA-1–expressing membrane in CLL T cells. PIP5KC (phosphatidylinositol-4-phosphate 5-kinase type I γ) and PtdIns(4,5)P2 (phosphatidylinositol-4,5-bisphosphate) are critical for LFA-1 affinity modulation downstream of RhoA and Rac. Diminished Rho GTPase activity and cytoskeletal signaling would suppress subsequent outside-in signaling and LFA-1–mediated adhesion and migration on the CD54 ligand. The ability of lenalidomide (red) to restore normal T-cell Rho GTPase activity and LFA-1 activation provides, for the first time, an important molecular definition of its mechanism of action. Cereblon expression is required for this immunomodulatory activity of lenalidomide. Dotted lines indicate that it is yet to be determined if lenalidomide activates Rho GTPases directly or indirectly via the cereblon pathway. These results indicate that lenalidomide represents a powerful immunotherapy to rescue global T-cell motility defects in cancer patients.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and donors who consented to use of their cell samples for this study. The authors also thank Dr. Shah-Jalal Sarker (Centre for Experimental Cancer Medicine) for statistical method advice and Celgene Corporation for the supply of lenalidomide. The authors are very grateful to Dr. Michael Way, Cancer Research UK, London Research Institute (London, UK) for providing the GFP-tagged Cdc42 V12 construct.
This work was supported by the European Hematology Association (The Hague, The Netherlands) (fellowship grant 2009/16) (A.G.R.), the National Cancer Institute to the CLL Research Consortium (program grant P01 CA81538) (J.G.G.), and Cancer Research UK (grant C1574/A6806).
Authorship
Contribution: A.G.R. designed, performed, and supervised experiments and their analyses and wrote the manuscript. R.E. designed and performed experiments and assisted in writing the manuscript. L.S. and S.K. assisted with experiments and their analysis. N.H. assisted in project direction and manuscript writing. J.G.G. designed and supervised experiments and their analyses and wrote the manuscript.
Conflict-of-interest disclosure: J.G.G. has received honoraria from Celgene Corporation (Summit, NJ) for work on advisory boards. The remaining authors declare no competing financial interests.
Correspondence: Alan G. Ramsay, Centre for Haemato-Oncology, Barts Cancer Institute, Charterhouse Square, London, EC1M 6BQ, United Kingdom; e-mail: a.ramsay@qmul.ac.uk.
References
Author notes
A.G.R. and R.E. contributed equally to this study as did N.H. and J.G.G.