Abstract
Indolent systemic mastocytosis (SM) patients have a varied clinical presentation, ranging from predominantly cutaneous symptoms to recurrent systemic symptoms (eg, flushing, palpitations, dyspepsia, diarrhea, bone pain) that can be severe and potentially life threatening (anaphylaxis). Mastocytosis patients without skin involvement pose a diagnostic challenge; a high index of suspicion is needed in those with mast cell–degranulation symptoms, including anaphylaxis following Hymenoptera stings or other triggers. Modern-era molecular and flow-cytometric diagnostic methods are very sensitive and can detect minimal involvement of bone marrow with atypical/clonal mast cells; in some cases, full diagnostic criteria for SM are not fulfilled. An important aspect of treatment is avoidance of known symptom triggers; other treatment principles include a stepwise escalation of antimediator therapies and consideration of cytoreductive therapies for those with treatment-refractory symptoms. The perioperative management of mastocytosis patients is nontrivial; a multidisciplinary preoperative assessment, adequate premedications, and close intra- and postoperative monitoring are critical. Smoldering mastocytosis is a variant with high systemic mast cell burden. While its clinical course can be variable, there is greater potential need for cytoreductive therapies (eg, interferon-alpha, cladribine) in this setting. A systematic approach to the diagnosis and treatment of indolent SM using a case-based approach of representative clinical scenarios is presented here.
Introduction
Mastocytosis results from a clonal proliferation of morphologically and immunophenotypically abnormal mast cells. The clinical presentation is heterogenous, ranging from skin-limited disease (cutaneous mastocytosis [CM]), particularly in children, to varying degrees of extracutaneous involvement (systemic mastocytosis [SM]), generally seen in adults. The World Health Organization (WHO) recognizes several SM variants (Table 1).1 Indolent SM (ISM) is predominantly characterized by symptoms related to mast cell degranulation/mediator release and/or allergies or anaphylaxis.2 In contrast, aggressive mastocytosis variants (aggressive SM, mast cell leukemia) are characterized by organ dysfunction related to mast cell infiltration.2,3 Some mastocytosis patients present concurrently with, or subsequently develop, an associated clonal hematological nonmast cell lineage disease, generally a myeloid neoplasm.4 Most adult mastocytosis patients, regardless of disease subtype, harbor the KITD816V mutation, which has pathogenetic and diagnostic relevance.2,5
This review focuses on relevant clinical issues in adult patients with ISM, including those with a high systemic mast cell burden (ie, smoldering SM [SSM]), using a case-based approach of representative clinical scenarios.
Indolent SM: Case 1
A 49-year-old male presented with skin rash that started on the trunk and gradually extended to the neck, upper arms, and thighs. A hyperpigmented macule had been biopsied 6 months prior, which revealed urticaria pigmentosa. Since then, the patient noted gradual onset of pruritus, flushing, and abdominal cramping with diarrhea. The pruritus had become severe and was triggered or exacerbated by heat. Episodes of severe flushing were triggered by alcohol consumption. The patient was nonatopic and denied a history of anaphylaxis, syncope, angioedema, or aspirin hypersensitivity. He reported a possible large local reaction to a bee sting several years ago. He denied gastroesophageal reflux or peptic ulcer symptoms. On examination, the patient was noted to be flushed but did not have lymphadenopathy or organomegaly; a positive Darier’s sign was elicited.
Does the patient have extracutaneous or systemic disease? What is the standard diagnostic workup in this setting?
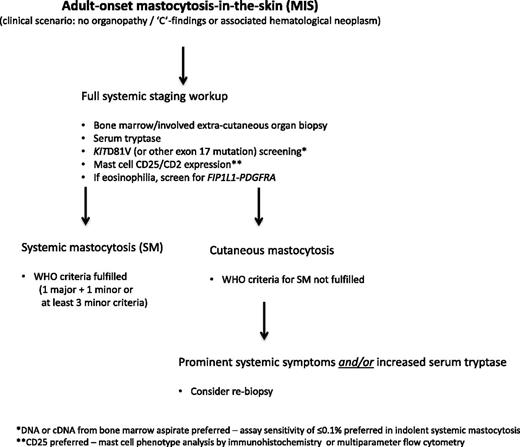
This patient has “mastocytosis in the skin” (MIS)6 ; this is an operational prediagnostic term pending completion of the staging workup to establish whether he has SM or CM (algorithm, Figure 1). Urticaria pigmentosa is more accurately referred to as maculopapular cutaneous mastocytosis (MPCM) and is the most common form of MIS in adults. It presents as symmetrically distributed red–brown macules or papules with the highest density of lesions on the trunk, whereas the palms, soles, face, and head are often spared.7,8 Mechanical irritation may cause reddening and urticarial swelling of the lesions via a release of mast cell mediators, the so-called Darier’s sign, which is pathognomonic for MIS. MPCM lesions rarely, if ever, resolve spontaneously in adults and may increase in density over time. The dermal mast cell infiltrate is revealed by (immuno)staining with toluidine blue, tryptase, or c-Kit/CD117, which reveals an average four- to five-time increase in spindled mast cells in the dermis, particularly in perivascular locations.8 Analysis of DNA from lesional skin reveals KITD816V in most adult cases but in less than half of pediatric cases.9,10
Allowing for differences based on referral patterns (dermatology vs allergy or hematology clinics), most patients with adult-onset MIS have demonstrable bone marrow (BM) involvement with clonal mast cells when modern-era diagnostic tools are used, in most instances, satisfying WHO diagnostic criteria for SM (Table 2).1,11 While historical series of patients with MIS revealed an 18% to 50% prevalence of systemic involvement based on conventional histologic criteria,12-15 more modern series suggest that only a minority of adult patients have skin-limited disease.10,16 Further, approximately 50% of adults with apparent skin-limited mastocytosis may have a clonal BM mast cell infiltrate that falls short of the diagnostic threshold for SM (satisfies major criterion only or only 1 or 2 minor criteria), suggesting prediagnostic or early stage of ISM.16 Consequently, it is highly probable that this patient has ISM. In our series of 342 SM patients, the majority (63%) with ISM had MIS.2
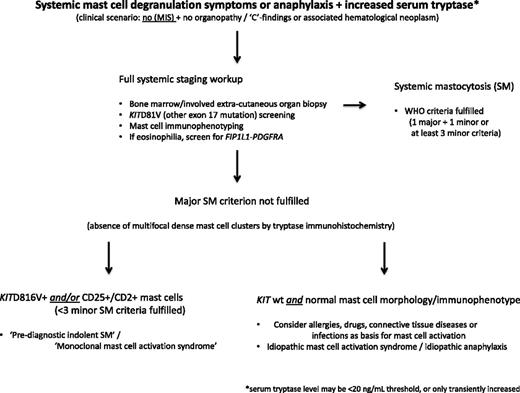
In general, the diagnosis of SM in the absence of MIS is considerably more challenging, particularly in those patients with an ISM variant with low mast cell burden, termed isolated BM mastocytosis.17 Consequently, a high index of suspicion is required in the setting of recurrent, unexplained anaphylaxis, flushing, osteoporosis, gastrointestinal ulcerative disease, or chronic abdominal cramping (algorithm, Figure 2). Some have suggested a stepwise diagnostic approach where the initial step is to document mast cell activation and, if confirmed, to then screen for KITD816V (or other KIT exon 17 mutation).18 If both tests are positive, a full diagnostic workup for mastocytosis including BM biopsy is indicated. At least 2 predictive algorithms applicable to patients with mast cell activation symptoms in the absence of MIS have been proposed to identify those patients with high likelihood of SM.19,20 Neither have been independently validated and consequently have limited clinical utility at the present time.
Diagnostic algorithm for patients with systemic mast cell degranulation symptoms or anaphylaxis and increased serum tryptase level, but without MIS.
Diagnostic algorithm for patients with systemic mast cell degranulation symptoms or anaphylaxis and increased serum tryptase level, but without MIS.
I pursue full diagnostic testing including BM biopsy in any patient in whom SM is strongly suspected (Figures 1 and 2). This is because 20% to 30% of SM patients have serum tryptase levels below the WHO-defined threshold of 20 ng/mL (sensitivity 80%, specificity 98%).21,22 Further, the sensitivity of KITD816V detection in peripheral blood is suboptimal, and tests for non-KITD816V mutation screening may not be readily available.
Laboratory studies were unremarkable. The serum tryptase level was 21.2 ng/mL (normal <11.5 ng/mL), urine β prostaglandin F2-α was 3540 ng/24 hours (normal ≤1000 ng/24 hours), and urine N-methylhistamine was 290 μg/g creatinine (normal 30–200 μg/g creatinine). BM biopsy revealed a normocellular marrow; mast cells were slightly increased (5%) and were distributed interstitially (no clusters). The mast cells were uniformly spindled and expressed CD25. Testing for KITD816V in peripheral blood was negative.
Does the patient meet diagnostic criteria for systemic mastocytosis? Does absence of the KITD816V mutation exclude this diagnosis?
I strictly follow WHO criteria for diagnosing SM (Table 2),1,11 while recognizing that a subset of patients with early or low burden disease may not fully meet the diagnostic threshold. While the patient lacked the pathognomonic mast cell clusters (major criterion), 3 minor criteria (ie, spindled morphology in >25% mast cells, CD25 expression on mast cells, and serum tryptase >20 ng/mL) were fulfilled, which is sufficient for diagnosing SM.
Attempts at validating the WHO diagnostic criteria reveal that approximately 20% of ISM patients lack mast cell clusters in the BM and approximately 30% exhibit a serum tryptase level < 20 ng/mL.16 In contrast, the sensitivity for detecting morphologic atypia, aberrant CD25, and/or CD2 expression, or KITD816V in BM mast cells, exceeds 90% when sensitive assays are used, thereby illustrating the increasing importance of these specific minor criteria in diagnosing SM.16,23 Patients who have mast cell degranulation symptoms with clonal mast cells (ie, mutated KIT gene and/or CD25 expression), but who do not meet criteria for SM (only 1 or 2 minor criteria satisfied, and no MIS), may have prediagnostic ISM or monoclonal mast cell activation syndrome; these patients generally have a normal or slightly elevated baseline serum tryptase level.18 While the clinical characteristics of this entity may be indistinguishable from ISM, its true natural history remains to be defined.
I prefer using DNA from BM aspirate for KITD816V screening given the low sensitivity of peripheral blood in this regard; I use our institutional allele-specific oligonucleotide polymerase chain reaction (sensitivity 0.01%) for mutation detection. Using this approach, we found 78% of ISM patients to harbor KITD816V.2 Although, the sensitivity of KITD816V detection may be higher when using sorted or purified mast cells, this option is not routinely available.5 Consequently, the inability to detect KITD816V in peripheral blood does not exclude SM given its restricted distribution in the myeloid compartment in ISM.5
How typical are the patient’s symptoms? How are they graded?
ISM patients can be highly symptomatic; in one study, 70% reported at least some degree of functional limitation, of which 17% reported severe limitation(s).24 Of the 38 symptoms scored, those most frequently associated with functional limitation were psychological impact of skin appearance, asthenia, pruritus, food allergy or intolerance, muscle/joint pain, drug allergy, pollakiuria, and dyspnea or bronchoreactivity (the same symptom spectrum has not been confirmed in other studies). Psychological and neurological symptoms, including depression, difficulty with social interactions, memory loss, headache, and reduced performance status, can also be significant contributors to functional limitation.24-26 Interestingly, the type and severity of symptoms were independent of disease classification (cutaneous vs SM), KITD816V status, and serum tryptase level (≥ 20 or < 20 ng/mL).24 The clinical severity of MIS can be assessed using the SCORMA (SCORing Mastocytosis) system; patients are scored across 3 fields including the extent of MIS, severity of representative cutaneous lesions, and symptom severity.27,28 Other criteria for grading MIS and cutaneous and systemic symptoms related to mast cell mediator release have also been proposed.6,29
I obtain a detailed inventory of symptoms, interrogate all major organ systems, and grade them on a scale of 0 to 4 (0, not present; 1, mild; 2, moderate; 3, severe; and 4, intolerable or requiring hospitalization or urgent intervention). This assessment is obtained at baseline and at regular intervals during follow-up.
How should this patient be managed?
The cornerstones of therapy are as follows:
Symptom trigger avoidance:
I closely question patients regarding potential symptom triggers that can be highly variable, including heat and humidity, emotional and physical stress, alcohol, medications (eg, aspirin, opioid analgesics, radiocontrast agents). I stress the importance of avoiding proven symptom triggers as much as possible.
Therapy of systemic mast cell mediator–release symptoms:
This chiefly relies on interventions that block the effects of mast cell mediators. However, there is a role for mast cell cytoreductive therapies, chiefly interferon-alpha or 2-chlorodeoxyadenosine (cladribine/2-CdA), in patients who are refractory to conventional therapies.30,31 I use antimediator drugs in a stepwise fashion, tailoring their use to the organ system affected (Table 3). H1- and H2-histamine receptor antagonists are most useful for alleviating pruritus and flushing and abdominal pain, heartburn, cramping, and diarrhea, respectively. I add proton pump inhibitors for patients with peptic ulcer disease or unresponsiveness to H2-antagonists.
Cromolyn sodium is a mast cell stabilizer that is useful for alleviating gastrointestinal symptoms.32 Leukotriene antagonists can be useful as adjunctive therapy in patients with suboptimal response to first-line antimediator drugs and may be steroid sparing in this role.33 Flushing can be severe and subjectively distressing and unresponsive to antihistamines. Aspirin can be useful in this setting, likely due to its ability to inhibit mast cell prostaglandin-D2 synthesis.34 While the optimal aspirin dose is unknown, maintenance doses of 81 to 500 mg twice daily have been shown to normalize 11β-prostaglandin-F2α excretion.35 There may be reluctance to use aspirin in mastocytosis patients given its ability to trigger anaphylaxis in some patients (particularly, if there is no history of prior safe aspirin use) and concerns regarding gastrointestinal toxicity at higher doses. If aspirin treatment is pursued, it must be undertaken in a closely supervised setting, using a graded dosing schedule.35 Despite reports of symptom improvement and reduction in mast cell mediator levels in ISM,36,37 imatinib mesylate, a tyrosine kinase inhibitor (TKI), likely has a limited role in treating unselected patients, since most patients harbor the imatinib-resistant KITD816V mutation.5 The rare cases that harbor an imatinib-sensitive KIT mutation or those who are KITD816 wild type may be appropriate candidates for imatinib treatment.37-41 Masitinib is an investigational TKI with in vitro inhibitory activity against wild-type KIT and juxtamembrane-activating mutations of KIT, but not KITD816V, that improves flushing, pruritus, and depression in mastocytosis patients. It remains unclear, however, as to what advantage, if any, this drug has over imatinib.26,42 A short course of corticosteroids is frequently effective in controlling symptoms that are refractory to other conservative measures.
Evaluation and treatment of allergy and anaphylaxis:
The prevalence of atopic disorders in mastocytosis is reportedly similar to that of the general population.29,43,44 In one study, 24% of mastocytosis patients had allergy; these patients were significantly more likely to be male and exhibit higher total immunoglobulin E (IgE) levels than those without allergy.44 Treatment of allergies in mastocytosis should follow general guidelines, with the exception of allergen immunotherapy, for which there are scant data regarding safety and efficacy (except Hymenoptera venom allergy; see below).
The incidence of anaphylaxis in adult mastocytosis patients ranges from 20% to 49%,44-46 which is significantly higher than the general population. Anaphylaxis is significantly more frequent in ISM, particularly in the absence of MIS, as compared with CM. In our study of 159 ISM patients, anaphylactoid reactions were significantly more frequent (86%) in those with BM mastocytosis.17 The anaphylaxis reaction is severe in half the patients and associated with loss of consciousness in a third; case reports of severe or fatal anaphylaxis have been published. Anaphylaxis may result from nonimmunologic or immunologic (IgE-mediated) mechanisms; major triggers are Hymenoptera stings, foods, and medication; in 17% to 37% of patients, no definite cause is identified. Mastocytosis patients with anaphylaxis are more likely to develop hypotension or circulatory collapse than asthma or laryngeal edema.47 In one study, virtually all patients had a baseline serum tryptase level >11.4 ng/mL, with a majority exhibiting levels >20 ng/mL.46 Patients with recurrent, unexplained anaphylaxis without MIS and normal or slightly increased tryptase levels may have clonal mast cells (without pathognomonic clusters) in the BM. This can occur in up to 40% of patients.48 Omalizumab, a humanized murine anti-IgE monoclonal antibody, may be effective in treating recurrent, treatment-refractory anaphylaxis in atopic and nonatopic mastocytosis patients, including in the context of ongoing Hymenoptera venom immunotherapy.49-51
Given the high incidence of mastocytosis in patients with unexplained anaphylaxis, I seriously consider a BM biopsy in such patients. I provide all mastocytosis patients with a prescription for self-injectable epinephrine (Epi-Pen) and train them and their caregivers in its use, regardless of anaphylactic history. Patients with definite anaphylaxis should wear a medical identification bracelet and have available at all times an emergency kit that contains at least 2 doses of self-administered epinephrine, rapid-actingH1 antihistamine (eg, diphenhydramine 2 25-mg tablets), and corticosteroid (prednisolone 2 50-mg tablets).
Perioperative management:
Mastocytosis patients who undergo surgical procedures may develop severe and potentially fatal cardiovascular shock in the absence of, or rarely despite of, adequate premedication (reviewed elsewhere52 ). Mast cell degranulation may occur immediately after trigger application; however, the risk may persist for several hours due to delayed mast cell mediator release.53,54 In the case of immediate reactions, skin testing may reveal the incriminating agent if IgE mediated.55 Nonimmune triggers include pressure, extremes of temperature, trauma, exercise, psychological stress, and medications. The choice of analgesic and anesthetic agents is challenging. The role of preoperative provocative skin testing to identify potential drug allergens is unclear and is not routinely performed.52 Although the risks associated with specific analgesic and anesthetic agents and the value of preanesthetic medications have not been definitively established, expert recommendations for reducing the risk of anaphylaxis during anesthesia are available.56-58
The general guidelines I follow for perioperative management of mastocytosis patients are outlined inTable 4. Close coordination between the hematologist or allergist and members of the surgical and anesthesia teams is important.
Management of Hymenoptera venom allergy and anaphylaxis (HVA):
HVA is an IgE-mediated phenomenon; the presentation can range from large local reactions to anaphylaxis that may be fatal.59 In a study of 74 adult mastocytosis patients, the most common trigger for severe anaphylaxis was Hymenoptera stings.45 In another study, 12% of patients with HVA, the majority with anaphylaxis, had an elevated baseline serum tryptase level.60,61 Clonal mast cells were detected in 88% of patients (8% of the overall cohort; approximately two-thirds had ISM and the remaining had lower levels of involvement, satisfying < 3 minor diagnostic criteria) when sensitive diagnostic methods were used. These data, among others, illustrate the strong association between HVA and mastocytosis.62 Measurement of baseline serum tryptase level may be a useful screening test for mastocytosis in patients with systemic reactions after Hymenoptera stings. However, a significant but variable proportion of patients will have levels <20 ng/mL, which creates a dilemma in terms of the cutoff level for BM biopsy.59 A positive correlation between baseline serum tryptase and grade of the initial allergic reaction has been reported.63 Sensitization to Hymenoptera venom can be detected by skin or serological tests. However, some have negative test results, possibly due to adsorption of circulating IgE to the increased mast cell bulk or due to a non-IgE mechanism for mast cell degranulation.64,65 Given the likelihood that HVA is IgE mediated in most mastocytosis patients, venom immunotherapy has been proposed as a treatment of choice, albeit with important caveats.59,66-68 General guidelines for the diagnosis and management of HVA are available, although they are not specific to mastocytosis patients.69
I pursue full investigation for SM including BM biopsy in patients with anaphylaxis after Hymenoptera stings, regardless of tryptase level or presence of MIS. Anaphylaxis precautions are as described above. A discussion of venom immunotherapy and other details is beyond the scope of this article. I refer relevant patients to an allergist with expertise in this area.
Evaluation and treatment of bone disease:
Patients frequently report bone pain; in one study, the prevalence was 54% (18% reported severe or intolerable symptoms).24 In contrast, the prevalence of bone involvement using objective criteria has been less well studied.70 In a study of 75 adult mastocytosis patients, 37 (49%) had bone involvement—23% had osteoporosis (17% with vertebral fracture), 8% osteosclerosis, and 4% a mixed pattern.71 In a study limited to ISM patients, 83 (57%) of 154 patients developed 235 lifetime fractures; 140 fractures in 57 (37%) patients were characterized as osteoporotic.72 An additional 43 (28%) patients had osteoporosis without fractures; overall, 78 (51%) patients had osteoporotic fracture and/or osteoporosis. Predictors of osteoporosis included older age, male gender, and higher urinary methylhistamine levels.
Development of osteoporosis may be related to release of histamine and/or proinflammatory cytokines (eg, interleukin 1 [IL-1], IL-6, and tumor necrosis factor-alpha) that affect bone metabolism.73,74 Studies of markers of bone turnover suggest increased bone remodeling and correlation with serum tryptase in some studies but not others.75,76 Early institution of antimediator therapy may reverse some disease-related bone changes;77 however, there are no definitive studies in this regard. Bisphosphonate therapy is effective in increasing vertebral bone mineral density (BMD; ∼2% per year)71 and preventing osteoporotic fractures, but has considerably less efficacy in increasing hip and femoral neck BMD.71,78-80 IFN-α is also effective in treating mastocytosis-related bone pain and osteoporosis, including in patients who develop new vertebral fractures despite bisphosphonate treatment.81-84
I obtain spine radiographs and measure BMD at the spine and hip by dual-energy x-ray absorptiometry (DXA) scans in all ISM patients. I ensure that all patients have an adequate intake of calcium and vitamin D. I optimize antimediator therapy in every case and institute bisphosphonate therapy when osteoporosis is documented. I follow updated clinical practice guidelines for bisphosphonate use in multiple myeloma.85-87 In severe and treatment-refractory cases, I consider the addition of cytoreductive therapy in the form of IFN-α or 2-CdA. I typically perform a DXA scan once every year until stability is achieved, with less frequent monitoring thereafter.
SSM: Case 2
A 61-year-old male presented with a 2-year history of fatigue, intermittent night sweats, and 15-pound voluntary weight loss. Physical examination revealed hyperpigmented macules and mild splenomegaly. Laboratory studies were remarkable only for a hemoglobin level of 12 g/dL (normal 13.5-17.5 g/dL) and serum alkaline phosphatase level of 149 U/L (normal 45-115 U/L). The serum tryptase level was 517 ng/mL (normal <11.5 ng/mL). Computed tomography imaging confirmed mild splenomegaly, and bone survey revealed innumerable scattered sclerotic lesions. BM trephine biopsy revealed a hypercellular marrow (80%) with panhyperplasia. Immunohistochemistry revealed atypical CD25+ mast cells in clusters comprising 40% to 50% of total BM cellularity. Megakaryocytes were quantitatively normal; however, a subset was hypolobate, and erythroid precursors were mildly megaloblastoid. Increased reticulin fibers (grade 1+) and osteosclerosis were noted. The BM karyotype was 46,XY, del (20) (q11.2q13.3) [1]/46,XY [19]. Testing for KITD816V was positive.
Diagnostic dilemma: does the patient have smoldering SM, aggressive SM, or SM with an associated clonal hematological non-MC lineage disease (SM-AHNMD)?
In order to subclassify patients who otherwise unambiguously satisfy diagnostic criteria for SM (Table 2), I first exclude mast cell leukemia (≥20% mast cells in BM aspirate smear) and SM-AHNMD. The latter is facilitated via integrated review of clinical, laboratory, and BM morphologic findings and discussion with the hematopathologist in difficult cases. While there was suggestion of an evolving chronic myeloid neoplasm on the basis of borderline morphology and cytogenetic findings in this case, it did not clearly meet WHO diagnostic criteria for the same.11 Next, I exclude aggressive SM. In this case, a C finding (Table 2) reflecting organopathy from mast cell infiltration was not identified; the weight loss was volitional and could not be attributed to mastocytosis-related malabsorption or protein losing enteropathy. Consequently, the patient was classified as having ISM. He satisfied criteria for the provisional WHO category of SSM on the basis of high mast cell burden, since all B findings (Table 2) were satisfied.
Prognostic dilemma: what is the risk of disease progression? What is the impact on life expectancy?
While patients with ISM comprise a heterogeneous group, their overall prognosis is relatively good as compared with other SM subgroups (Figure 3). In our study of 342 SM patients, those with ISM comprised the largest subgroup (159 patients, 46%) and were significantly younger at presentation (median age 49 years).2,17 The overall median survival was not significantly different than that of the age- and gender-matched control population. Advanced age was the primary determinant of inferior survival, and the overall risk of transformation to acute leukemia or ASM was low (<1% and 3%, respectively). A low rate of disease progression (1.7% ± 1.2% at 5-10 years and 8.4% ± 5% at 20-25 years) and excellent overall survival (2.2% ± 1.3% deaths at 5 years and 11% ± 5.9% deaths at 25 years) were also confirmed in another study.88 The majority of deaths in this ISM cohort were unrelated to mastocytosis; presence of KITD816V in all hematopoietic lineages was independently associated with disease progression.
Overall survival of systemic mastocytosis patients. Kaplan-Meier survival curves for SM patients per WHO subtype: indolent SM (ISM; red curve); aggressive SM (ASM; green curve); SM with AHNMD (AHD; yellow curve); and mast cell leukemia (MCL; purple curve). Survival of SM patients is compared with survival of age- and gender-matched US population (blue curve). Previously published in Blood.2
Overall survival of systemic mastocytosis patients. Kaplan-Meier survival curves for SM patients per WHO subtype: indolent SM (ISM; red curve); aggressive SM (ASM; green curve); SM with AHNMD (AHD; yellow curve); and mast cell leukemia (MCL; purple curve). Survival of SM patients is compared with survival of age- and gender-matched US population (blue curve). Previously published in Blood.2
The risk of disease progression and leukemic transformation (18%) may be higher in SSM as compared with other ISM subgroups. The significantly inferior overall survival of SSM patients was largely accounted for by their older age.17
Given the high prevalence of osteoporotic vertebral fractures in ISM patients, it has been argued that presence of such fractures should not automatically classify patients as having “aggressive systemic mastocytosis,” since not all such fractures may qualify as a C finding per WHO criteria and the risk is modifiable with therapy.16,71,72 In my view, this issue merits further prospective study.
Therapeutic dilemma: observation vs cytoreductive vs investigational therapy?
I manage SSM patients conservatively given that the natural history of this condition has not been clearly defined and the clinical course and prognosis can be variable. Further, SSM has not been shown to be biologically distinct on the basis of specific mutations that render it more amenable to treatment with molecularly targeted drugs. Consequently, in the absence disease-modifying therapies, I manage SSM patients as I would those with other forms of ISM (treatment principles as outlined for case 1), while recognizing that there is a greater potential need for cytoreductive therapies in this setting. SSM patients have a higher incidence of constitutional symptoms, anemia, and/or symptomatic hepatosplenomegaly,17 features that are poorly responsive to antimediator therapies alone. For progressive symptoms that are unresponsive to conservative measures, I add IFN-α as the first-line cytoreductive agent, given its efficacy in improving mediator-release and gastrointestinal symptoms, skin rash, osteoporosis, cytopenias, and ascites and hepatosplenomegaly, with corresponding decrease in BM mast cell burden in some cases (reviewed elsewhere83,89,90 ). Since treatment tolerability is a major issue, I generally “start low and go slow.” I start treatment at the dose of 1 to 3 MU subcutaneously 3 times per week, followed by gradual escalation to 3 to 5 MU for 3 to 5 times per week, as tolerated. Prednisone (30-60 mg/day), which can be added at the start of treatment to improve tolerability and response, is tapered over a 2- to 3-month period. IFN-α treatment is generally continued as long as a response is observed and there are no intolerable adverse effects.
If a more rapid cytoreduction is needed or if intolerance or refractoriness to IFN-α is documented, I switch treatment to 2-CdA.91-94 The conventional dose is 5 mg/m2/day or 0.13 to 0.15 mg/kg/day as a 2-hour infusion for 5 days every 4 to 8 weeks, generally for up to 6 cycles, depending on efficacy and tolerability (reviewed elsewhere90 ). Potential toxicities of 2-CdA include myelosuppression and lymphopenia with increased risk of opportunistic infections. I use prophylaxis against Pneumocystis jirovecii infection for a minimum of 3 months after completion of therapy or until the CD4+ T-lymphocyte count is >200/μL, whichever occurs later. To further minimize the infectious risk, a lower dose intensity (5 mg/m2 once weekly for 4 weeks, followed by once every 2 to 4 weeks) has been explored in a few ISM patients with treatment-refractory symptoms; data regarding efficacy and tolerability of this approach are awaited (A. Tefferi, personal communication). Midostaurin (PKC412) is an investigational multitargeted TKI that inhibits both KIT wild type and KITD816V and has shown activity in treating advanced SM, with significant decrease in serum tryptase and BM mast cell burden in 40% to 50% of cases.95 If shown to be relatively safe with longer-term use, midostaurin may have a role in the treatment of indolent and smoldering mastocytosis.
Authorship
Contribution: A.P. is the sole author and is responsible for the content of this manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Animesh Pardanani, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: pardanani.animesh@mayo.edu.