In this issue of Blood, Dovedi et al demonstrate convincing evidence for the therapeutic efficacy of a new systemic immunostimulatory agent, the toll-like receptor-7 (TLR7) agonist R848, to augment radiotherapy-induced anticancer immunity.1
A growing armamentarium of nonspecific cytotoxic agents and increasingly sophisticated radiotherapy protocols have proven inadequate for the treatment of the majority of cancer patients and spurred research into immunotherapy to complement conventional approaches. The immune system has a central role in suppressing tumor growth, and its subversion is now accepted as a key component of cancer pathophysiology.2,3 Why does this massively potent inbuilt defensive system fail and allow the cancer to establish? How can it be redirected and augmented as a therapeutic measure? And does conventional cytotoxic chemotherapy in fact reduce the potency of the endogenous defense system by suppressing its key components, leukocytes, at the point at which they are most required?
Two approaches demonstrate the effectiveness of immunotherapy in hemato-oncology, confirming the potency of a redirected, augmented immune response. First, there is clear evidence that a major component of the curative potential of allogeneic hematopoietic stem cell transplantation comes from the graft-versus-tumor effect. Second, monoclonal antibody therapy, such as rituximab, has single-agent activity, and chemo-immunotherapy has become the standard of care for many B-cell malignancies. However, one huge advance in immunotherapy has failed to translate to the setting of cancer treatment. After the profound impact in public health through treatment of infectious disease, vaccination strategies seemed a natural clinical progression of a burgeoning understanding of the interactions among tumor cells, their infiltrating microenvironment, and the host immune response. However, despite decades of promising preclinical research, this has failed to translate into meaningful clinical results.4 The failure of cancer vaccination, along with many trials of adoptive and bioengineered T-cell therapy, only recently entering into early phase clinical trials,5 the emergence of rituximab-refractory B-cell malignancies, and the frequent relapses seen in allogeneic transplant recipients have shown that significant work still has to be done in identifying the means by which cancer escapes and suppresses the immune response. The key to stimulating long-lasting, effective anticancer immunity lies in generating a potent tumor-specific cytotoxic response, augmented by Th1-biased lymphocytes unimpeded by a less effective, competing Th2 bias and unopposed by regulatory T cells, through effective, anergy-bypassing peptide presentation by appropriately co-stimulated antigen presenting cells, and subsequent robust immunologic memory without exhaustion resulting from chronic stimulation.
Systemic immunostimulatory agents, predominantly acting through inhibiting immunosuppressive pathways, such as anti–CTLA-46 or anti-PD1/PD-L1 mAbs,7,8 are already showing promise in early-phase clinical trials. TLR therapies target one of the fundamental on-switches of innate immunity: recognition of pathogen-/damage-associated molecular patterns (PAMPs/DAMPs). Dovedi et al present convincing evidence for the efficacy of a combination of systemic TLR7-agonist administration (R848) with radiotherapy, an established treatment modality that is finding a new role in immunotherapy.
The potency of the deleterious immune response of graft-versus-host disease (GVHD) is potentiated by cellular damage arising from conditioning regimes, particularly those incorporating radiotherapy.9 As well as inducing immunostimulatory molecule expression and cytokine secretion in exposed tissue, irradiation forms free radicals, modifies and degrades intracellular proteins, and consequently augments the presentation of a broader peptide repertoire.10 While deleterious in the context of allogeneic transplant, where the immune response is directed to healthy tissue to produce GVHD, if induced specifically on tumor cells this effect may contribute to some of the therapeutic efficacy of radiotherapy—not only destroying cancer cells directly through DNA damage, but also immuno-sensitizing through modified antigen presentation.
Dovedi et al take a methodical and comprehensive approach, not only demonstrating the efficacy of combined systemic immunostimulation and local radiotherapy, but also determining its mechanism. Using a well-characterized immunocompetent murine model of T-cell malignancy, they confirm modest efficacy of R848 administered without radiotherapy, but no evidence of any directly cytotoxic or radiation-sensitizing effect of the drug in vitro. The strength of this paper lies in a logical series of carefully designed experiments, which isolate the precise immunologic mechanism for R848's indirect cytotoxic action: augmentation of antigen presentation, effector lymphocyte generation, and memory T-cell maintenance (see figure). R848-treated mice showed elevation of levels of key cytokines, particularly IL6, IFNγ, and TNFα, implying that effective systemic immunostimulation was at work. In turn depleting B cells, CD4+ T-helper cells, and CD8+ cytotoxic T cells, the authors confirm that only CD8+ cell depletion abrogates the drug's effectiveness, going on to demonstrate that more tumor-specific CTLs are generated in the combined therapy–exposed mice than in tumor-innoculated untreated mice. They show enhancement of costimulatory B7 molecule expression, CD80 and CD86, on dendritic cells after exposure to the drug in vitro. Memory responses are confirmed in long-surviving mouse splenocyte coculture experiments, with multiple tumor-specific antigen targets responsible. Finally, and perhaps most intriguingly, they find that manipulating the radiotherapy regimen from the more intuitively tumor-toxic single dose to a less toxic fractionated dose regimen in fact enhances the efficacy of combined therapy, impressively leading to the eradication of a less immunogenic cell line in all mice.
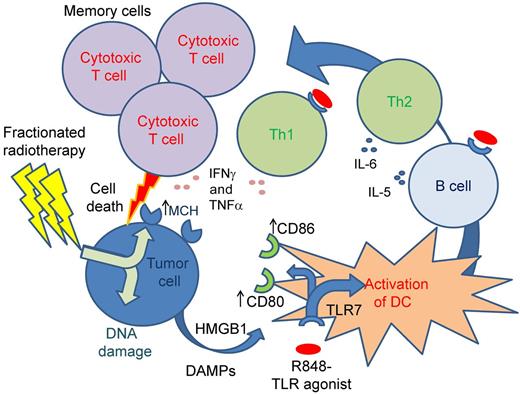
Schematic representation of mechanism of cancer cell immune killing and induction of T-cell memory. Irradiation of tumor cells induces DNA damage, cellular apoptosis, and release of HMGB1. In combination with the toll-like receptor agonist R848, this results in increased antigen-presenting capacity of dendritic cells (DCs). This in combination with R848 activates effector CD4 T cells and B cells, but in this model leads specifically to effector CD8 T cells that kill the tumor cells. An important component is the induction of antigen-specific T-cell memory protecting mice from subsequent cancer challenge.
Schematic representation of mechanism of cancer cell immune killing and induction of T-cell memory. Irradiation of tumor cells induces DNA damage, cellular apoptosis, and release of HMGB1. In combination with the toll-like receptor agonist R848, this results in increased antigen-presenting capacity of dendritic cells (DCs). This in combination with R848 activates effector CD4 T cells and B cells, but in this model leads specifically to effector CD8 T cells that kill the tumor cells. An important component is the induction of antigen-specific T-cell memory protecting mice from subsequent cancer challenge.
Their comprehensive mechanistic approach lends credibility to this model as a future therapeutic strategy. The authors' assertion that multiple antigenic targets are responsible, on the basis of superior memory T-cell responses to coculture with whole tumor cell versus soluble tumor-specific antigen, may be overstated because the context of antigen presentation in priming (cell-surface, MHC-associated versus soluble) is clearly important in determining the potency of a subsequent immune response. In addition, any convenient “single immune cell compartment” model (in this case CD8+ mediated, not CD4+ or CD20+) is likely an oversimplification based on the use of homogenized inbred mouse strains and innoculated cell lines versus endogenously developing tumors. As always, the murine model awaits first-in-human trials to show that this exciting innovation is not another immunotherapeutic disappointment. However, Dovedi and colleagues have elegantly demonstrated mechanistic and therapeutic efficacy of a logical 2-pronged approach to immunotherapy: enhance immunogenicity of tumor through radiotherapy, enhance systemic immunity through a potent “immune danger” signal, and let the body's own defenses do the rest.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■