Key Points
MPO, via its catalytic activity, inhibits the generation of adaptive immunity by suppressing DC function.
MPO-mediated inhibition of adaptive immunity attenuates T cell-driven tissue inflammation.
Myeloperoxidase (MPO) is important in intracellular microbial killing by neutrophils but extracellularly causes tissue damage. Its role in adaptive immunity and T-cell−mediated diseases is poorly understood. Here, T-cell responses in lymph nodes (LNs) were enhanced by MPO deletion or in vivo inhibition, causing enhanced skin delayed-type hypersensitivity and antigen (Ag)-induced arthritis. Responses of adoptively transferred OT-II T cells were greater in MPO-deficient than wild-type (WT) recipients. MPO, deposited by neutrophils in LNs after Ag injection, interacted with dendritic cells (DCs) in vivo. Culture of murine or human DCs with purified MPO or neutrophil supernatant showed that enzymatically dependent MPO-mediated inhibition of DC activation occurs via MPO-generated reactive intermediates and involves DC Mac-1. Transfer of DCs cultured with WT, but not MPO-deficient, neutrophil supernatant attenuated Ag-specific immunity in vivo. MPO deficiency or in vivo inhibition increased DC activation in LNs after immunization. Studies with DQ-ovalbumin showed that MPO inhibits Ag uptake/processing by DCs. In vivo DC transfer and in vitro studies showed that MPO inhibits DC migration to LNs by reducing their expression of CCR7. Therefore, MPO, via its catalytic activity, inhibits the generation of adaptive immunity by suppressing DC activation, Ag uptake/processing, and migration to LNs to limit pathological tissue inflammation.
Introduction
Myeloperoxidase (MPO) is an enzyme found in azurophilic granules of neutrophils.1 It is the major neutrophil protein, accounting for approximately 5% of the dry cell weight.1 MPO is also found to a much lesser extent in monocytes and some macrophages.1,2 In the presence of hydrogen peroxide (H2O2) and a halide (chloride [Cl], bromide [Br], thiocyanate [SCN]), tyrosine, or nitric oxide (NO), MPO catalyses the formation of powerful reactive intermediates, including hypochlorous (HOCl), hypobromous (HOBr), and hypothiocyanous (HOSCN) acids, tyrosyl radical and reactive nitrogen species, respectively, which can have profound biological effects by modifying proteins, lipids, and/or DNA.1,2
MPO plays an important role in intracellular pathogen killing, but when it is released extracellularly after neutrophil activation, it can cause tissue damage at inflammatory sites, as demonstrated in patients with immune and inflammatory diseases, including cardiovascular disease, rheumatoid arthritis, atherosclerosis, kidney disease, cystic fibrosis, and multiple sclerosis (MS).1,2 Studies in models of cardiovascular, renal, and lung disease3,-5 also demonstrate that MPO is a local mediator of tissue injury. These studies have implicated MPO as an important therapeutic target, leading to great interest in the design of MPO inhibitors for the treatment of inflammatory diseases. However, very little is known about the effects of MPO on adaptive immunity and T-cell−driven diseases. The mechanisms by which MPO affects adaptive immune responses also are unknown.
Neutrophils, the principal source of MPO, have long been considered solely as first responders in innate immunity. However, increasing evidence suggests that they also influence the development of adaptive immune responses.6 Neutrophils interact with dendritic cells (DCs) at inflammatory sites7 and secrete mediators that promote DC maturation.8,9 In addition to infiltrating inflamed tissue, neutrophils migrate to draining lymph nodes (LNs) after antigen (Ag) injection or infection.10,11 However, it is not very clear how neutrophils in LNs affect adaptive immunity. Moreover, although neutrophils contain molecules such as NO,12 which regulates effector T cells at inflammatory sites and can, by unknown pathways, suppress the induction of T-cell responses,13 the mechanisms by which neutrophils may dampen adaptive immunity are poorly understood.
In these studies, the generation of CD4+ T-cell responses in LNs was enhanced by MPO deletion or in vivo inhibition, resulting in exacerbated dermal delayed-type hypersensitivity (DTH) and Ag-induced arthritis (AIA). We hypothesized that MPO attenuates the initiation of T-cell responses by affecting DCs. This study shows that MPO is deposited by neutrophils in LNs, where it interacts with DCs during the induction of adaptive immunity. We demonstrate that MPO, via an enzymatically dependent formation of reactive intermediates, inhibits the generation of adaptive immune responses by limiting DC activation, Ag uptake/processing, and migration to LNs.
Methods
Animals
We obtained 8- to 10-week old C57BL/6J wild-type (WT) and CD45.1+ male mice from Monash University Animal Services (Clayton, Victoria, Australia). MPO knockout (Mpo−/−) mice were backcrossed onto C57BL/6J background for ≥10 generations.14 OT-II mice were from B. Heath (Walter and Eliza Hall Institute, Parkville, Victoria, Australia). Mice were bred/kept at Monash Medical Centre Animal Facilities (Clayton, Victoria, Australia) under specific pathogen-free conditions. Studies were approved by the Monash University Ethics Committee and conducted in accordance with the Declaration of Helsinki.
Immunizations, in vivo MPO inhibition, and AIA
Mice were injected subcutaneously with ovalbumin (OVA; 0.5 mg)/complete Freund’s adjuvant (CFA; Sigma-Aldrich) or OVA (0.5 mg)/lipopolysaccharide (LPS, 30 μg; Sigma-Aldrich). For in vivo MPO inhibition, mice received 4-aminobenzoic acid hydrazide (ABAH; 13 μg/g; Sigma-Aldrich)15,16 or dimethylsulfoxide (DMSO: vehicle; Sigma-Aldrich) subcutaneously.
For AIA, mice were immunized subcutaneously (inguinal) with methylated bovine serum albumin (mBSA, 0.5 mg; Sigma-Aldrich)/CFA (day [d]0), boosted (d7) with mBSA (0.2 mg; axillary)/incomplete Freund’s adjuvant (Sigma-Aldrich) and injected intra-articularly (d12) with mBSA (30 μg; left knee) or saline (vehicle; right knee). For OVA-AIA, mice received OVA (0.5 mg)/CFA subcutaneously (d0) and OVA (30 μg; left knee) or saline (right knee) intra-articularly (d7). Arthritis was assessed on d19 (mBSA-AIA) or d12 (OVA-AIA). Formalin-fixed paraffin-embedded Safranin-O−stained sagittal joint sections were scored 0-3 for: joint tissue inflammation (leukocytes in infrapatellar fat pads, joint capsule, and area adjacent to periosteal sheath), exudate (leukocytes in joint space), and synovitis (synovial hypercellularity). For OVA-AIA, tissue inflammation and synovitis were assessed. The total arthritis score was generated by adding the aforementioned parameters.
Flow cytometry and confocal microscopy
Proliferation, apoptosis, and T- and B-cell activation were assessed by intracellular bromodeoxyuridine incorporation (or Ki-67 staining [anti-Ki-67/fluorescein SCN (FITC), clone 35; BD biosciences]), Annexin-V staining, and expression of CD44 and CD69, respectively.5 Regulatory T cells were identified as foxp3+CD25+CD4+ cells.17 Mouse DCs were stained with anti-CD11c/phycoerythrin (PE) (BD biosciences), anti-CD86/allophycocyanin (APC)-Cy7 (Biolegend), anti-CD40/FITC (BD biosciences), anti-CCR7/APC (eBioscience), and anti-major histocompatibility complex (MHC)-II/APC (Biolegend). Human DCs were stained with anti-CD1a/APC (BD biosciences), anti-CD14/PE (Miltenyi), anti-CD86/APC (BD biosciences), and anti-HLA-DR FITC (Biolegend). Murine neutrophils were identified as CD11bhiCD11c−MHC-IIneg side-scatterhi cells that were Ly-6G+ or Gr-1hi using anti-CD11b/APC-Cy7 (BD biosciences), anti-CD11c/PE, anti-MHC-II/APC, anti-Ly-6G/PE (1A8; BD biosciences), and anti-Gr-1/Alexa647 (RB6-8C5; DNAX). For CD68 expression, cells were surface-stained with anti-CD68/FITC (FA/11; Biolegend). Flow cytometry was performed using the MoFlo flow cytometer (Summit software; Dako). Frozen LN sections were stained with anti-Ly6G/PE (BD biosciences), anti-CD11c/APC (BD biosciences), and anti-MPO/FITC (Thermo-Scientific) and analyzed by confocal microscopy.
Cytokines, DTH, serum antibody, and MPO activity
Mouse cytokines were measured by enzyme-linked immunosorbent essay (ELISA).17 Intracellular cytokine staining was performed as published.18 Human interleukin (IL)-12p70 was assessed by ELISA by the use of anti-human IL-12p70 (BD biosciences), biotin anti-human IL-12p40 (Biolegend), and recombinant human IL-12p70 (BD biosciences). DTH was assessed by measuring thickness of footpads injected with OVA or BSA.5 Serum antibodies were measured by ELISA5 with the use of OVA-coated plates. MPO activity was measured as published.5
In vitro experiments
Murine bone marrow (BM)-derived DCs were generated as described.8 Nonadherent cells (d8) were enriched for DCs (MoFlo XDP cell sorter, Beckman Coulter; >99% CD11chi). Neutrophils, obtained by sorting anti-Gr-1/Alexa647-labeled BM cells (>95% purity; cytospin DIFF-Quik staining [Laboratory Aids, New South Wales, Australia]), were incubated with cytochalasin-B (CB, 5 μg/mL, 30 min; Sigma-Aldrich), then N-formyl-methionyl-leucyl-phenylalanine (fMLP; 10 μM, 1 hour; Sigma-Aldrich),19 ± ABAH (200 μM) and supernatant collected. DCs were cultured (18 hours; in 10% RPMI containing 108 mM Cl) with LPS (1 μg/mL), H2O2 (100 μM; Merck) and neutrophil supernatant or purified enzymatically active native mouse MPO20 ± ABAH (200 μM). CB, fMLP, ABAH, or DMSO alone did not affect DC activation (not shown). In some experiments, DCs were cultured with LPS/H2O2 (with/without mouse MPO ± ABAH) and with (i) Cl alone, Cl + NaBr (100 μM; Sigma-Aldrich), Cl + NaSCN (200 μM; Sigma-Aldrich) or Cl + sodium nitroprusside (SNP; 10 μM; Sigma-Aldrich), or (ii) anti-Mac-1 antibody (5C6; 10 μg/mL; Walter and Eliza Hall Institute)21 or rat IgG (10 μg/mL).
Cell transfer experiments
CD4-enriched, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled naive OT-II cells (Vα2+Vβ5+; OVA323-339-specific) were transferred intravenously (3 × 106/mouse) to mice, as described.18 OVA/CFA or OVA/LPS was injected subcutaneously 2 days later. OT-II responses were measured in LNs/spleen after 72 hours.
CD45.1+ DCs (2.5 × 106/mouse; +OVA/LPS) were injected subcutaneously into CD45.2+ recipients and detected in LNs by flow cytometry with anti-CD45.1/FITC (eBioscience).
DCs cultured with LPS/H2O2 and with/without neutrophil supernatant or mouse MPO ± ABAH were loaded with OVA323-339 (1 μM; Auspep, West Melbourne, Australia) and injected subcutaneously into recipients (1 × 106/mouse). Two mice received uncoated DCs. Mice received naive OT-II cells (5 × 106 Vα2+Vβ5+CD4+/mouse intravenously) 2 days before DC transfer. OVA-specific responses were assessed 9d after DC injection.
Ag uptake/processing
DCs were cultured (2 hours) with DQ-OVA (10 μg/mL; Invitrogen) and LPS/H2O2 and with/without purified mouse MPO and analyzed by flow cytometry. Mice were injected subcutaneously with DQ-OVA (50 μg)/LPS and anti-CD11c/PE-labeled LN cells analyzed by flow cytometry 4 hours later.
Experiments with human cells
Venous blood from healthy volunteers was centrifuged over Polymorphprep (Axis-Shield, Oslo, Norway) to obtain peripheral blood mononuclear cells and neutrophils. Peripheral blood mononuclear cell were enriched (anti-CD14-microbeads; Miltenyi Biotec) to obtain CD14+ monocytes (>90% purity), which were cultured with recombinant human granulocyte macrophage–colony-stimulating factor (GM-CSF, 800 U/mL; R&D Systems) and IL-4 (500 U/mL; R&D Systems). On d6, cells were >95% CD1a+ (DCs) and <1% CD14+. Neutrophils (>98% pure; cytospin DIFF-Quik) were incubated with human GM-CSF (1000 U/mL; 1 hour), then fMLP (10 μM; 1 hour)22 ± ABAH (100 μM) or catalase (2000 U/mL; Sigma-Aldrich) and supernatant collected. DCs were cultured (24 hours) with LPS (1 μg/mL)/H2O2 (100 μM) and with/without human neutrophil supernatant or purified human MPO (Sigma-Aldrich).
Statistics
Results are expressed as mean ± standard error of the mean. Unpaired t test and one-way analysis of variance (with the Bonferroni or Dunnett test) were used to compare means between 2 or >2 groups, respectively. Statistical analysis was performed with GraphPad Prism (GraphPad Software). Significance was indicated by P < .05.
Results
MPO suppresses the generation of adaptive immune responses
MPO deficiency increased CD4+ T-cell activation and proliferation in LNs and spleen 6 days after the injection of OVA/CFA (Figure 1A-B). T-cell activation/proliferation was not different between nonimmunized WT and Mpo−/− mice (supplemental Table 1; see the Blood Web site). T-cell apoptosis and regulatory T cells were not affected by MPO deletion (supplemental Table 2). OVA-stimulated splenocytes from Mpo−/− mice secreted more interferon (IFN)-γ and IL-17A (Figure 1C), as well as IL-4 (1.6 ± 0.2 pg/mL vs 11.3 ± 1.7 pg/mL; P < .001) and Ag-presenting cell-derived IL-12 (15.2 ± 5.8 pg/mL vs 33.1 ± 5.6 pg/mL, P < .05). MPO deletion enhanced OVA-specific IFN-γ and IL-17A (Figure 1D-E) but not IL-4 (0.48 ± 0.05% vs 0.49 ± 0.05% IL-4+ CD4 cells; 10.1 ± 0.3 vs 10.7 ± 0.3 mean fluorescence intensity) production by CD4+ T cells. The lack of MPO did not affect IFN-γ but enhanced IL-17A production by CD4− cells (supplemental Figure 1). IL-4 was undetected in CD4− cells. Increased T-cell responses in LNs/spleen correlated with enhanced T-cell−mediated skin DTH in Mpo−/− mice (Figure 1F).
MPO deficiency enhances T-cell responses leading to increased skin DTH and AIA. (A-F) WT (n = 8) and Mpo−/− mice (n = 8) were immunized subcutaneously with OVA/CFA and CD4+ T-cell activation (A) and proliferation (B) assessed in LNs and spleen 6 days later (flow cytometry). (C) Concentrations of cytokines in 72-hour supernatants from OVA-stimulated splenocytes (ELISA). (D-E) Intracellular staining for IFN-γ and IL-17A in OVA-stimulated CD4-labeled splenocytes. Spleen cells were cultured (48 hours) in the presence of OVA (10 μg/mL) with brefeldin-A (5 μg/mL) added for the last 8 hours. CD4-labeled cells were then stained intracellularly for IFN-γ and IL-17A. The percentage of CD4+ T cells producing IFN-γ and IL-17A (Di), and IFN-γ and IL-17A expression by CD4+ T-cell (expressed as mean fluorescence intensity; Dii) are shown. (E) Representative flow cytometry plots showing IFN-γ (Ei) and IL-17A (Eii) production by CD4+ T cells from WT and Mpo−/− mice. (F) Skin DTH, expressed as the difference in thickness of footpads injected with OVA (right footpad) or BSA (left footpad). (G) CD4-enriched CFSE-labeled naïve OT-II T cells were transferred intravenously to WT (n = 4) and Mpo−/− recipient mice (n = 4) on d0. Recipient mice were injected subcutaneously with OVA/CFA on d2. (Gi) Proliferation of OT-II T cells in draining LNs of recipient mice 72 hours after OVA/CFA immunization (based on CFSE dilution; flow cytometry). (Gii-iii) IFN-γ production by OT-II T cells in recipient mice (intracellular staining of CD4/Vβ5-labeled splenocytes cultured with OVA323-339 [1 μM]). (Hi) The development of mBSA-induced AIA in WT (n = 8) and Mpo−/− (n = 9) mice. (Hii) Representative photomicrographs of formalin-fixed, paraffin-embedded 3-μm-thick Safranin-O-stained (fast green/hematoxylin counterstain) joint sections of WT and Mpo−/− mice with mBSA-AIA showing more severe disease in MPO-deficient animals. Sections were examined on a Leica BMLB Laboratory microscope with a ×5/0.70 NA objective lens. Images were captured with a Leica DC 300F digital camera using Photoshop CS software (Adobe Systems). Original magnification, ×50. Data are representative of 2-3 independent experiments. *P < .05, **P < .01, ***P < .001, •P = .07, #P = .1.
MPO deficiency enhances T-cell responses leading to increased skin DTH and AIA. (A-F) WT (n = 8) and Mpo−/− mice (n = 8) were immunized subcutaneously with OVA/CFA and CD4+ T-cell activation (A) and proliferation (B) assessed in LNs and spleen 6 days later (flow cytometry). (C) Concentrations of cytokines in 72-hour supernatants from OVA-stimulated splenocytes (ELISA). (D-E) Intracellular staining for IFN-γ and IL-17A in OVA-stimulated CD4-labeled splenocytes. Spleen cells were cultured (48 hours) in the presence of OVA (10 μg/mL) with brefeldin-A (5 μg/mL) added for the last 8 hours. CD4-labeled cells were then stained intracellularly for IFN-γ and IL-17A. The percentage of CD4+ T cells producing IFN-γ and IL-17A (Di), and IFN-γ and IL-17A expression by CD4+ T-cell (expressed as mean fluorescence intensity; Dii) are shown. (E) Representative flow cytometry plots showing IFN-γ (Ei) and IL-17A (Eii) production by CD4+ T cells from WT and Mpo−/− mice. (F) Skin DTH, expressed as the difference in thickness of footpads injected with OVA (right footpad) or BSA (left footpad). (G) CD4-enriched CFSE-labeled naïve OT-II T cells were transferred intravenously to WT (n = 4) and Mpo−/− recipient mice (n = 4) on d0. Recipient mice were injected subcutaneously with OVA/CFA on d2. (Gi) Proliferation of OT-II T cells in draining LNs of recipient mice 72 hours after OVA/CFA immunization (based on CFSE dilution; flow cytometry). (Gii-iii) IFN-γ production by OT-II T cells in recipient mice (intracellular staining of CD4/Vβ5-labeled splenocytes cultured with OVA323-339 [1 μM]). (Hi) The development of mBSA-induced AIA in WT (n = 8) and Mpo−/− (n = 9) mice. (Hii) Representative photomicrographs of formalin-fixed, paraffin-embedded 3-μm-thick Safranin-O-stained (fast green/hematoxylin counterstain) joint sections of WT and Mpo−/− mice with mBSA-AIA showing more severe disease in MPO-deficient animals. Sections were examined on a Leica BMLB Laboratory microscope with a ×5/0.70 NA objective lens. Images were captured with a Leica DC 300F digital camera using Photoshop CS software (Adobe Systems). Original magnification, ×50. Data are representative of 2-3 independent experiments. *P < .05, **P < .01, ***P < .001, •P = .07, #P = .1.
Recipient Mpo−/− mice had increased proliferation of adoptively transferred OT-II T cells in LNs (Figure 1Gi) and spleen (supplemental Figure 2) in response to immunization with OVA/CFA or OVA/LPS (not shown). OT-II activation (33.2 ± 2.7% vs 45.2 ± 2.2% CD44hi cells; P < .01) and IFN-γ production (Figure 1Gii-iii) also were increased in Mpo−/− mice. MPO deficiency enhanced B-cell responses (supplemental Table 3) without reducing their apoptosis (not shown).
MPO attenuates AIA
To assess how MPO affects T-cell−driven pathological tissue inflammation, we induced AIA in WT and Mpo−/− mice. Mpo−/− mice had increased CD4+ T-cell activation (9.4 ± 1.1% vs 15.7 ± 1.1% CD44hi T cells; P < .01) and IFN-γ production (1778 ± 715 pg/mL vs 6570 ± 1482 pg/mL; P < .05) in response to mBSA. In correlation with enhanced arthritogenic T-cell immunity, arthritis severity was augmented by MPO deletion (Figure 1Fi-ii). Similar results were obtained in OVA-induced AIA (supplemental Figure 3).
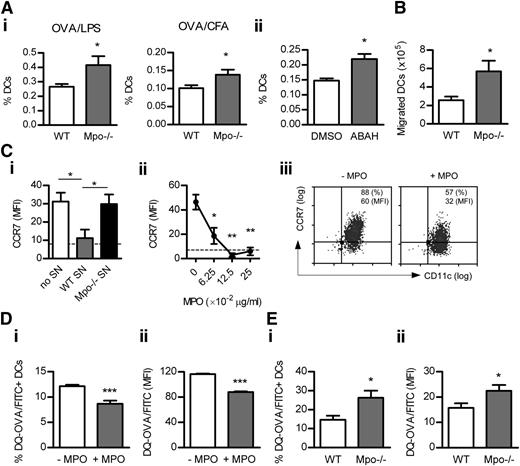
Neutrophil MPO interacts with DCs in LNs
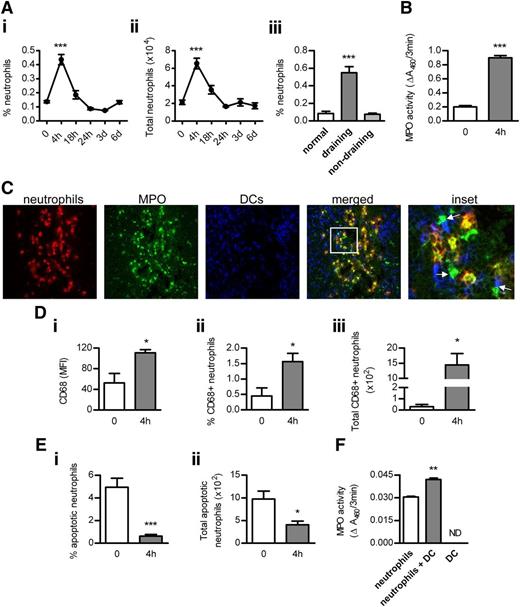
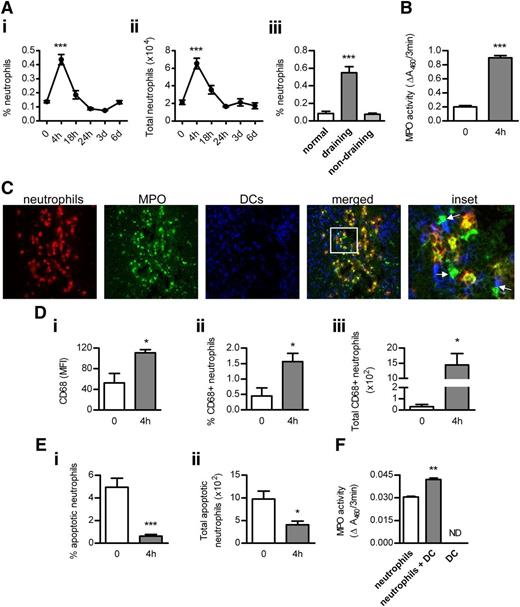
Neutrophil infiltration was increased in draining, but not nondraining, LNs after OVA/LPS injection, peaking at 4 hours and decreasing thereafter (Figure 2A). Correlating with neutrophil accumulation, MPO activity was increased in draining LNs at 4 hours (Figure 2B). Neutrophil recruitment in draining LNs before or 4 hours after OVA/LPS immunization was not affected by MPO deficiency (0 hours: 0.06 ± 0.01 vs 0.06 ± 0.03; 4 hours: 0.51 ± 0.03% vs 0.56 ± 0.04% neutrophils). Numerous contacts were observed between neutrophils and DCs in LNs at 4 hours (Figure 2C). Importantly, MPO was detected not only inside but also outside of neutrophils, and multiple contacts were observed between extracellular MPO and DCs (Figure 2C).
The interaction between neutrophils, MPO, and DCs in LNs. (Ai-ii) Accumulation of neutrophils (identified as Ly-6G+CD11bhiCD11c−MHC-IIneg side-scatterhi cells; flow cytometry) in draining LNs of normal (n = 6) and subcutaneously OVA/LPS-injected mice (n = 6/time point). (Aiii) Neutrophil infiltration in normal (n = 6) and draining (inguinal) or nondraining (axillary) LNs from mice injected with OVA/LPS (4 hours; n = 6). (B) MPO activity in extracts of draining LNs from normal and OVA/LPS-injected mice (4 hours; n = 6/group). (C) Frozen acetone-fixed sections (4-μm-thick) of draining LNs from WT mice collected 4 hours after OVA/LPS injection were stained for neutrophils (red), MPO (green), and DCs (blue). Confocal images were captured using a NikonC1 inverted confocal laser scanning microscope (×40/0.45 Nikon Plan Apo objective lens), acquired with the use of line sequential scanning (acquisition software: NIS Elements AR 3.0), converted to .TIFF files (Image J software) and processed by Photoshop CS software (Adobe Systems). Neutrophils interact with DCs (magenta). MPO is present inside (yellow) and outside (green) of neutrophils and extracellular MPO contacts DCs (indicated by arrows). Original magnification, ×400. (Di) Surface expression of CD68 (flow cytometry) on LN neutrophils and the proportion (Dii) and total number (Diii) of CD68+ neutrophils in draining LNs of normal (n = 6) and OVA/LPS-injected mice (4 hours; n = 6). (Ei-ii) Neutrophil apoptosis (Annexin-V staining; flow cytometry) in draining LNs of normal (n = 6) and OVA/LPS-injected mice (4 hours; n = 6). (F) The effect of DCs on MPO release by neutrophils. BM neutrophils were cultured (in 10% RPMI; 24 hours) with or without BM-derived DCs (1:1 ratio). Control wells contained DCs cultured alone. MPO activity was measured in supernatants. Data are representative of 2-3 independent experiments. *P < .05, **P < .01, ***P < .0001; ND, not detected.
The interaction between neutrophils, MPO, and DCs in LNs. (Ai-ii) Accumulation of neutrophils (identified as Ly-6G+CD11bhiCD11c−MHC-IIneg side-scatterhi cells; flow cytometry) in draining LNs of normal (n = 6) and subcutaneously OVA/LPS-injected mice (n = 6/time point). (Aiii) Neutrophil infiltration in normal (n = 6) and draining (inguinal) or nondraining (axillary) LNs from mice injected with OVA/LPS (4 hours; n = 6). (B) MPO activity in extracts of draining LNs from normal and OVA/LPS-injected mice (4 hours; n = 6/group). (C) Frozen acetone-fixed sections (4-μm-thick) of draining LNs from WT mice collected 4 hours after OVA/LPS injection were stained for neutrophils (red), MPO (green), and DCs (blue). Confocal images were captured using a NikonC1 inverted confocal laser scanning microscope (×40/0.45 Nikon Plan Apo objective lens), acquired with the use of line sequential scanning (acquisition software: NIS Elements AR 3.0), converted to .TIFF files (Image J software) and processed by Photoshop CS software (Adobe Systems). Neutrophils interact with DCs (magenta). MPO is present inside (yellow) and outside (green) of neutrophils and extracellular MPO contacts DCs (indicated by arrows). Original magnification, ×400. (Di) Surface expression of CD68 (flow cytometry) on LN neutrophils and the proportion (Dii) and total number (Diii) of CD68+ neutrophils in draining LNs of normal (n = 6) and OVA/LPS-injected mice (4 hours; n = 6). (Ei-ii) Neutrophil apoptosis (Annexin-V staining; flow cytometry) in draining LNs of normal (n = 6) and OVA/LPS-injected mice (4 hours; n = 6). (F) The effect of DCs on MPO release by neutrophils. BM neutrophils were cultured (in 10% RPMI; 24 hours) with or without BM-derived DCs (1:1 ratio). Control wells contained DCs cultured alone. MPO activity was measured in supernatants. Data are representative of 2-3 independent experiments. *P < .05, **P < .01, ***P < .0001; ND, not detected.
Neutrophils deposit MPO in LNs via primary granule release
Exposure of CD68 (which is expressed on primary granules of neutrophils) on the cell surface when granules fuse with the plasma membrane can be used as a marker of primary granule exocytosis.8 Surface expression of CD68 increased on a proportion of LN-infiltrating neutrophils 4 hours after OVA/LPS injection (Figure 2Di). The frequency and total number of CD68+ neutrophils were enhanced in LNs at 4 hours (Figure 2Dii-iii).
Aging of BM neutrophils demonstrated a positive association between neutrophil apoptosis and MPO release in vitro (supplemental Figure 4). However, at the time when extracellular MPO was detected in LNs (4 hours), only 0.6% of all LN-infiltrating neutrophils were apoptotic compared with 5% in normal LNs (Figure 2Ei). The total number of apoptotic neutrophils was reduced at 4 hours (Figure 2Eii). Similar to previous reports,11 increased neutrophil apoptosis was observed later, at 18 hours (6.4 ± 0.9% and 23.8 ± 6.1 × 102 total apoptotic neutrophils), correlating with neutrophil disappearance from LNs by 24 hours.
MPO suppresses DC activation in vitro through its catalytic activity
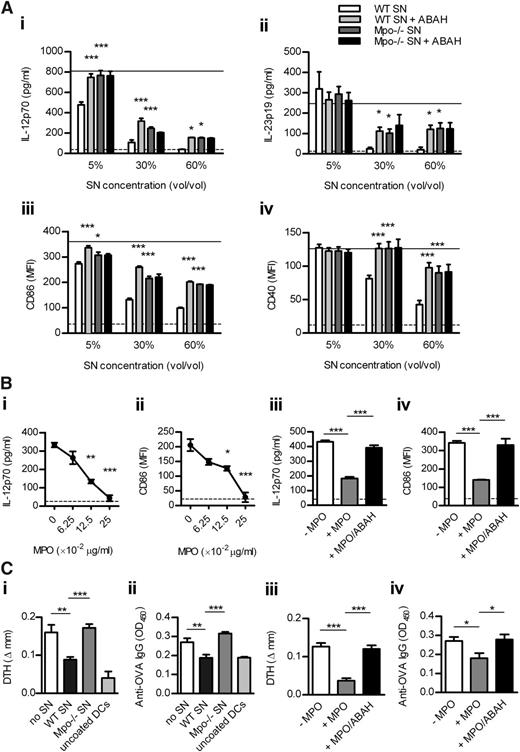
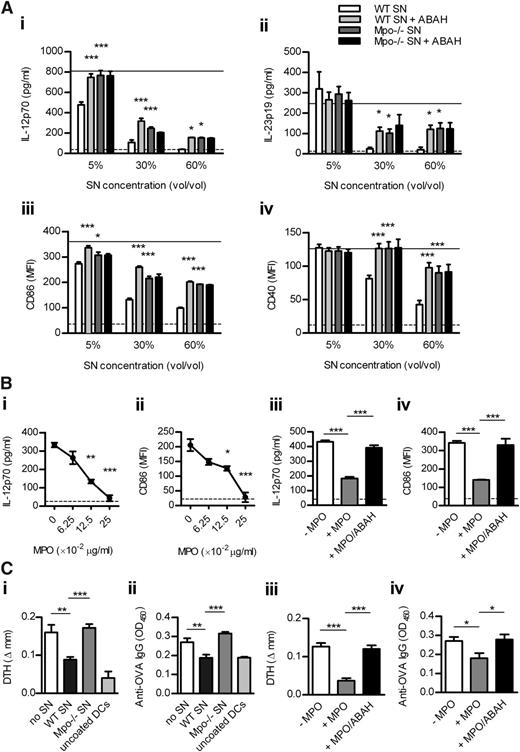
To examine whether MPO affects DC activation, we first performed in vitro experiments. WT and Mpo−/− neutrophils were degranulated in the presence or absence of the MPO inhibitor (ABAH) so we could test whether MPO-mediated effects on DCs are dependent on its catalytic activity. Enzymatically active MPO in supernatant from CB/fMLP-stimulated WT neutrophils was inhibited by ABAH and was undetectable in Mpo−/− supernatant (supplemental Table 4). In the presence of H2O2 (essential MPO substrate), WT supernatant dose-dependently inhibited LPS-induced DC activation (Figure 3A). WT supernatant containing ABAH and Mpo−/− supernatant reversed this effect, completely at lower and partially at higher supernatant doses. ABAH in Mpo−/− supernatant did not affect DC activation (Figure 3A), confirming its specificity. These effects of MPO were not the result of increased DC apoptosis (not shown). IL-12 and IL-23 were undetectable in neutrophil supernatants (not shown), indicating that the cytokines measured in DC/neutrophil-supernatant cultures were DC-derived. In addition, purified enzymatically active mouse MPO dose-dependently suppressed DC activation, effects that were lost when MPO was inhibited by ABAH (Figure 3B).
MPO from mouse neutrophils decreases DC activation in vitro leading to reduced adaptive immune response in vivo. (A) BM-derived murine DCs were cultured (18 hours) with LPS (1 μg/mL), H2O2 (100 μM), and increasing doses of supernatant from CB/fMLP (± ABAH)-stimulated WT or Mpo−/− neutrophils. DC activation was assessed by measuring production of IL-12 and IL-23 (ELISA) and expression of CD86 and CD40 (flow cytometry). Solid line represents DCs cultured with LPS/H2O2 only. Dotted line represents unstimulated DCs. (B) DCs were cultured with LPS/H2O2 and (Bi-ii) increasing concentrations of purified enzymatically active mouse MPO or (Biii-iv) with or without MPO (0.125 μg/mL) ± ABAH. (C) OVA323-339-coated DCs, which had been cultured with LPS/H2O2 and (Ci-ii) with or without supernatant (5% v/v) from CB/fMLP-stimulated WT or Mpo−/− neutrophils or (Ciii-iv) with or without purified MPO (0.125 μg/mL) ± ABAH, were injected subcutaneously into WT recipients (n = 6-8/group). Two recipients received uncoated DCs cultured only with LPS/H2O2. OVA-specific skin DTH and serum IgG (1/50 dilution; ELISA) were measured 9 days later. Data are representative of 2-3 independent experiments. Each group in (A-B) contained 4 replicates. *P < .05, **P < .01, ***P < .001. SN, supernatant.
MPO from mouse neutrophils decreases DC activation in vitro leading to reduced adaptive immune response in vivo. (A) BM-derived murine DCs were cultured (18 hours) with LPS (1 μg/mL), H2O2 (100 μM), and increasing doses of supernatant from CB/fMLP (± ABAH)-stimulated WT or Mpo−/− neutrophils. DC activation was assessed by measuring production of IL-12 and IL-23 (ELISA) and expression of CD86 and CD40 (flow cytometry). Solid line represents DCs cultured with LPS/H2O2 only. Dotted line represents unstimulated DCs. (B) DCs were cultured with LPS/H2O2 and (Bi-ii) increasing concentrations of purified enzymatically active mouse MPO or (Biii-iv) with or without MPO (0.125 μg/mL) ± ABAH. (C) OVA323-339-coated DCs, which had been cultured with LPS/H2O2 and (Ci-ii) with or without supernatant (5% v/v) from CB/fMLP-stimulated WT or Mpo−/− neutrophils or (Ciii-iv) with or without purified MPO (0.125 μg/mL) ± ABAH, were injected subcutaneously into WT recipients (n = 6-8/group). Two recipients received uncoated DCs cultured only with LPS/H2O2. OVA-specific skin DTH and serum IgG (1/50 dilution; ELISA) were measured 9 days later. Data are representative of 2-3 independent experiments. Each group in (A-B) contained 4 replicates. *P < .05, **P < .01, ***P < .001. SN, supernatant.
MPO-mediated suppression of DC activation inhibits immune responses in vivo
To show that MPO suppresses adaptive immunity via DCs, we transferred OVA323-339-coated DCs cultured with/without WT or Mpo−/− supernatant or with/without purified mouse MPO ± ABAH, into WT recipients. Compared with cells cultured with LPS/H2O2 alone, DCs cultured with WT supernatant (Figure 3Ci-ii) or purified MPO (Figure 3Ciii-iv) decreased OVA-specific DTH and serum IgG. This effect was reversed by DCs cultured with Mpo−/− supernatant or MPO + ABAH (Figure 3C).
MPO suppresses DC activation through various reactive intermediates
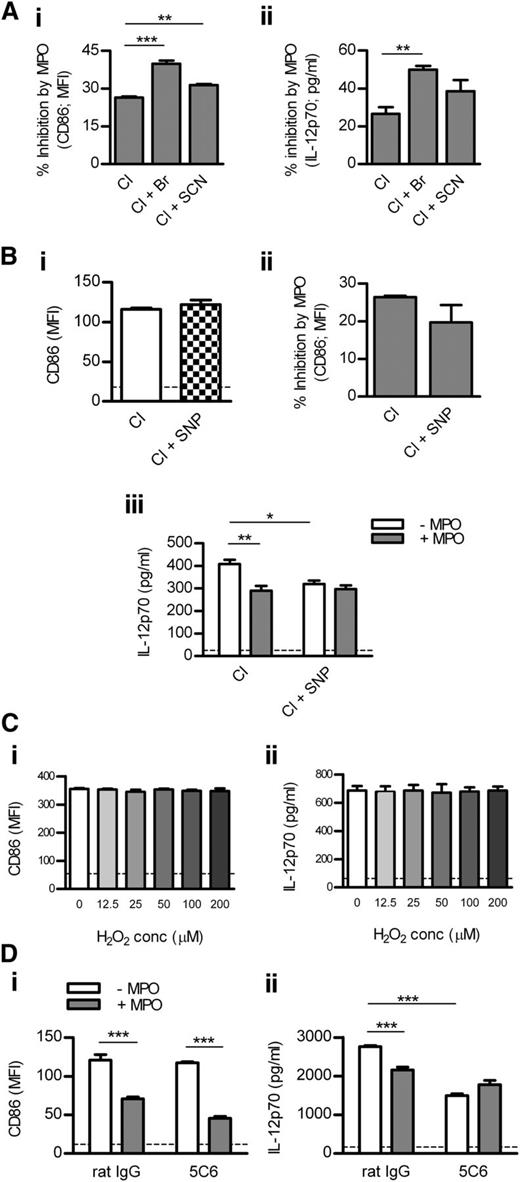
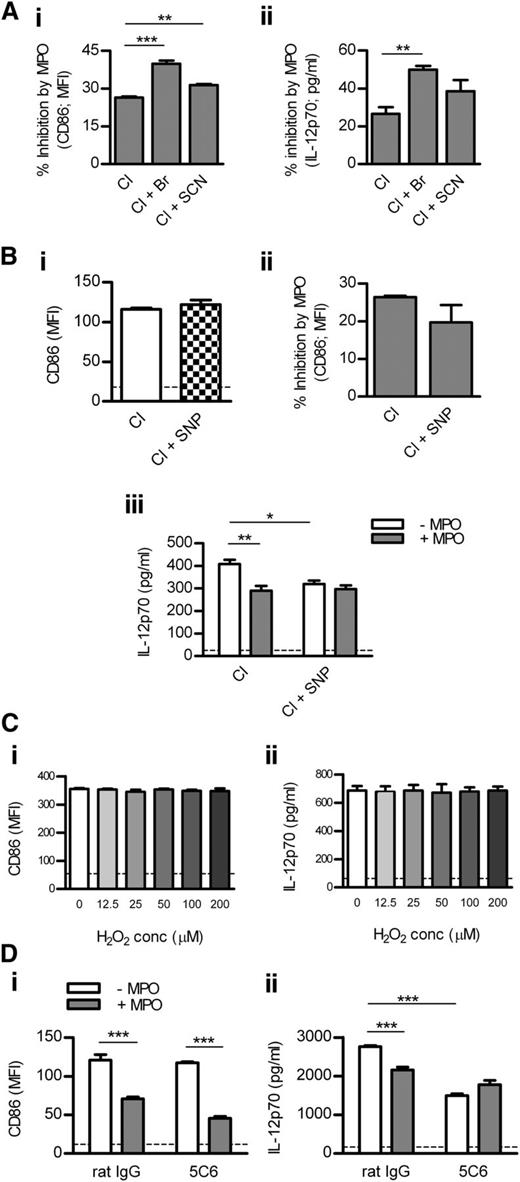
To examine how different MPO-generated oxidants affect DC activation, we cultured DCs with MPO in the presence of various halides. Compared with Cl alone, MPO-mediated inhibition of DC activation was enhanced in the presence of Cl + Br and, to a lesser extent, Cl + SCN (Figure 4Ai-ii). Br or SCN did not affect DC activation in the absence of MPO (not shown).
Mechanisms by which MPO inhibits DC activation. (A) BM-derived murine DCs were cultured (18 hours) with LPS (1 μg/mL), H2O2 (100 μM), and purified mouse MPO (0.125 μg/mL) in the presence of Cl alone, Cl + Br, or Cl + SCN. Percentage inhibition of DC CD86 (Ai; flow cytometry) and IL-12 expression (Aii; ELISA) by MPO was assessed. (Bi) CD86 expression by DCs cultured with LPS/H2O2 in the presence of Cl alone or Cl + SNP (NO donor). (Bii) DCs were cultured with LPS/H2O2 and mouse MPO (0.125 μg/mL) in the presence of Cl alone or Cl + SNP. Percentage inhibition of DC CD86 expression by MPO is shown. (Biii) IL-12 production by DCs cultured with LPS/H2O2 and with or without mouse MPO (0.125 μg/mL) in the presence of Cl alone or Cl + SNP. (C) CD86 and IL-12 expression by DCs cultured (18 hours) with LPS (1 μg/mL) and increasing concentrations of H2O2. (D) CD86 and IL-12 expression by DCs cultured with LPS/H2O2 and with or without mouse MPO (0.125 μg/mL) in the presence of rat IgG or ligating/activating anti-Mac-1 antibody (5C6). Data are representative of 2-3 independent experiments. All groups contained 4 replicates. Dotted line represents unstimulated DCs. *P < .05, **P < .01, ***P < .001.
Mechanisms by which MPO inhibits DC activation. (A) BM-derived murine DCs were cultured (18 hours) with LPS (1 μg/mL), H2O2 (100 μM), and purified mouse MPO (0.125 μg/mL) in the presence of Cl alone, Cl + Br, or Cl + SCN. Percentage inhibition of DC CD86 (Ai; flow cytometry) and IL-12 expression (Aii; ELISA) by MPO was assessed. (Bi) CD86 expression by DCs cultured with LPS/H2O2 in the presence of Cl alone or Cl + SNP (NO donor). (Bii) DCs were cultured with LPS/H2O2 and mouse MPO (0.125 μg/mL) in the presence of Cl alone or Cl + SNP. Percentage inhibition of DC CD86 expression by MPO is shown. (Biii) IL-12 production by DCs cultured with LPS/H2O2 and with or without mouse MPO (0.125 μg/mL) in the presence of Cl alone or Cl + SNP. (C) CD86 and IL-12 expression by DCs cultured (18 hours) with LPS (1 μg/mL) and increasing concentrations of H2O2. (D) CD86 and IL-12 expression by DCs cultured with LPS/H2O2 and with or without mouse MPO (0.125 μg/mL) in the presence of rat IgG or ligating/activating anti-Mac-1 antibody (5C6). Data are representative of 2-3 independent experiments. All groups contained 4 replicates. Dotted line represents unstimulated DCs. *P < .05, **P < .01, ***P < .001.
Because MPO can consume NO,2 we tested the effect of MPO on DCs in the presence or absence of the NO donor, SNP. SNP did not affect DC CD86 up-regulation in the absence of MPO (Figure 4Bi). Compared with Cl alone, MPO-mediated inhibition of CD86 expression was not affected in the presence of Cl + SNP (Figure 4Bii). However, SNP reduced IL-12 production by DCs in the absence of MPO, and MPO-mediated suppression of IL-12 production that occurred in the presence of Cl alone was completely lost when SNP was also present (Figure 4Biii).
Mac-1 is involved in MPO-mediated inhibition of DC activation
To examine whether DC Mac-1 is involved in MPO-mediated regulation of DC activation, we tested the effect of MPO on DCs in the presence or absence of anti-Mac-1 antibody (5C6). 5C6 blocks leukocyte adhesion via Mac-121 but affects DC activation in a similar manner as anti-Mac-1 (M1/70), which ligates/activates DC Mac-125 (supplemental Figure 5). 5C6 did not affect CD86 expression in DCs in the absence of MPO, and MPO-mediated inhibition of CD86 expression was not affected by 5C6 (Figure 4Di). However, 5C6 down-regulated IL-12 production by DCs in the absence of MPO (Figure 4Dii). MPO suppressed DC IL-12 production in the absence, but not in the presence, of the ligating anti-Mac-1 antibody (Figure 4Dii).
MPO decreases DC activation in vivo
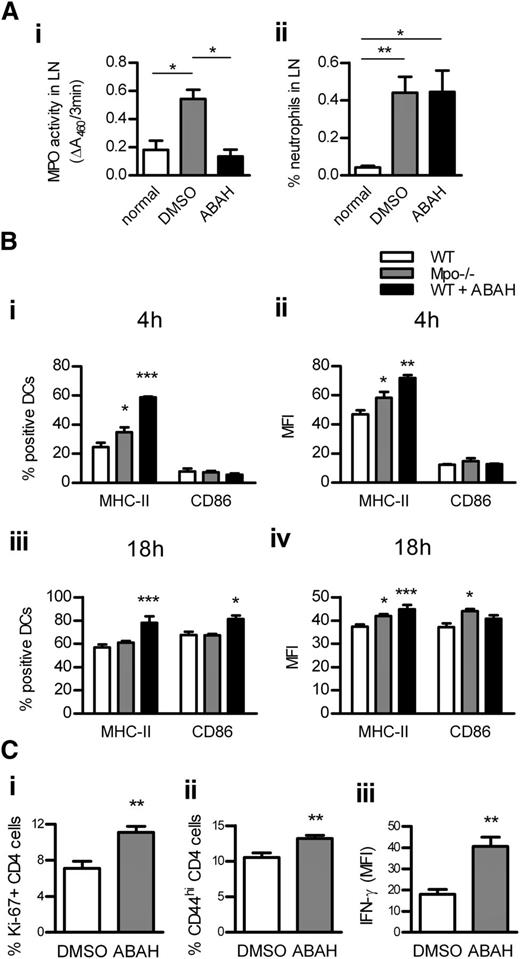
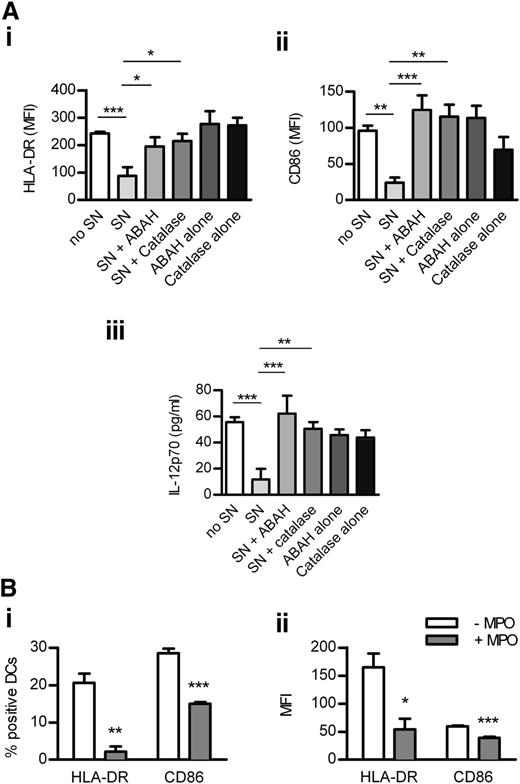
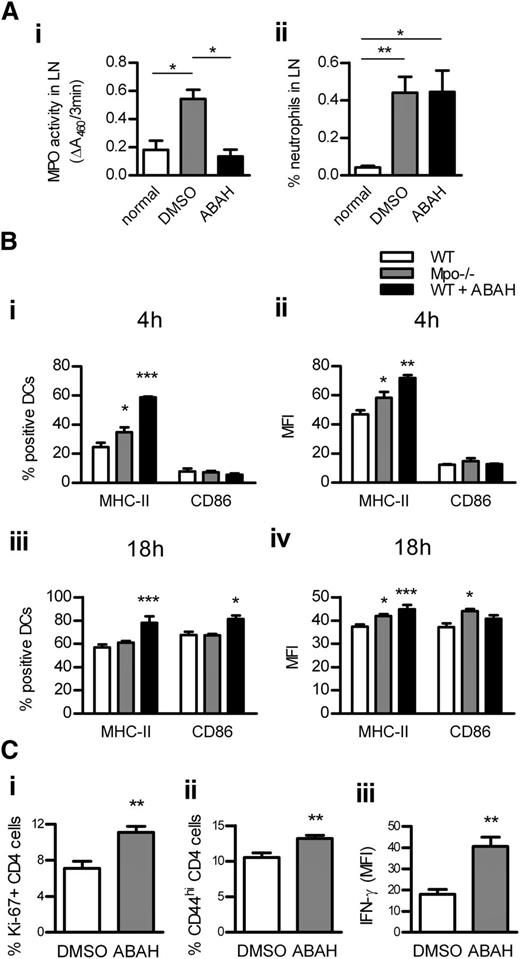
DC activation was assessed in LNs of WT and Mpo−/− mice 4 hours and 18 hours after OVA/LPS injection. To test whether in vivo MPO inhibition has a similar effect as MPO deletion, another group of WT mice received OVA/LPS/ABAH. ABAH reduced MPO activity in LNs to baseline levels without affecting neutrophil recruitment (Figure 5Ai-ii). MPO deletion and in vivo inhibition increased DC activation in LNs at both time points (Figure 5Bi-iv). DC activation was decreased in LNs of nonimmunized Mpo−/− mice (supplemental Table 5). In correlation with increased DC activation in LNs, T-cell responses were enhanced by in vivo administration of ABAH (Figure 5C). ABAH did not affect DCs or T cells in Mpo−/− mice, indicating its specificity (supplemental Table 6).
DC activation in LNs is increased by MPO deletion or in vivo inhibition. MPO activity (Ai; assessed by measuring ΔA460 upon oxidation of o-dianisidine dihydrochloride in the presence of H2O2) and neutrophil accumulation (Aii; flow cytometry) in draining LNs of normal (n = 4) and subcutaneously OVA/LPS-immunized WT mice receiving ABAH (n = 4) or DMSO (vehicle; n = 4). (B) DC activation (flow cytometry) 4 hours (Bi-ii) and 18 hours (Biii-iv) after subcutaneous OVA/LPS injection, in draining LNs of WT (n = 6/time point) and Mpo−/− mice (n = 6/time point), and WT mice receiving ABAH (n = 6/time point). CD4 T-cell proliferation (Ci; intracellular Ki-67 staining; flow cytometry), activation (Cii; CD44 expression; flow cytometry), and IFN-γ production (Ciii; intracellular staining, flow cytometry) 3 days after OVA/LPS injection, in draining LNs of WT mice receiving DMSO (n = 6) or ABAH (n = 6). Data are representative of 2 independent experiments. *P < .05, **P < .01, ***P < .001.
DC activation in LNs is increased by MPO deletion or in vivo inhibition. MPO activity (Ai; assessed by measuring ΔA460 upon oxidation of o-dianisidine dihydrochloride in the presence of H2O2) and neutrophil accumulation (Aii; flow cytometry) in draining LNs of normal (n = 4) and subcutaneously OVA/LPS-immunized WT mice receiving ABAH (n = 4) or DMSO (vehicle; n = 4). (B) DC activation (flow cytometry) 4 hours (Bi-ii) and 18 hours (Biii-iv) after subcutaneous OVA/LPS injection, in draining LNs of WT (n = 6/time point) and Mpo−/− mice (n = 6/time point), and WT mice receiving ABAH (n = 6/time point). CD4 T-cell proliferation (Ci; intracellular Ki-67 staining; flow cytometry), activation (Cii; CD44 expression; flow cytometry), and IFN-γ production (Ciii; intracellular staining, flow cytometry) 3 days after OVA/LPS injection, in draining LNs of WT mice receiving DMSO (n = 6) or ABAH (n = 6). Data are representative of 2 independent experiments. *P < .05, **P < .01, ***P < .001.
MPO inhibits DC migration to LNs
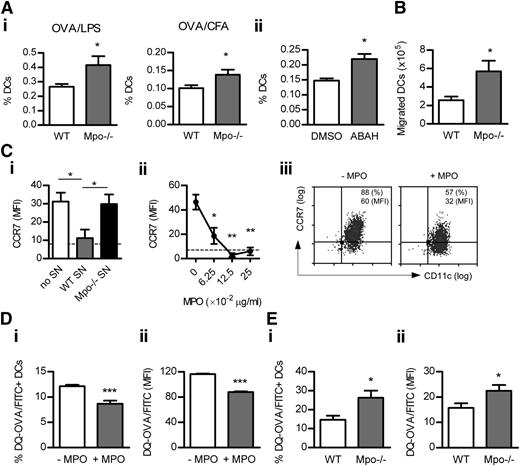
MPO deficiency or in vivo inhibition by ABAH augmented the frequency of endogenous DCs in LNs 18 hours after OVA/LPS or OVA/CFA injection (Figure 6A). The percentage of DCs in LNs of nonimmunized Mpo−/− mice was decreased (supplemental Table 5). ABAH did not affect the proportion of DCs in LNs of Mpo−/− mice (supplemental Table 6).
MPO suppresses DC Ag uptake/processing and migration to LNs. The frequency of DCs (CD11chi cells; flow cytometry) in draining LNs of WT (n = 8) and Mpo−/− mice (n = 8) 18 hours after subcutaneous OVA/LPS (Ai) or OVA/CFA (Aii) injection or (Aiii) OVA/LPS-injected WT mice receiving DMSO (n = 8) or ABAH (n = 8). (B) BM-derived CD45.1+ DCs (+OVA/LPS) were injected subcutaneously into CD45.2+ WT (n = 6) and Mpo−/− recipients (n = 6). Accumulation of the transferred DCs in draining LNs was assessed 18 hours later (flow cytometry). (C) CCR7 expression (flow cytometry) on BM-derived murine DCs cultured (18 hours) with LPS (1 μg/mL), H2O2 (100 μM) and (Ci) with or without supernatant (5% v/v) from CB/fMLP-degranulated WT or Mpo−/− neutrophils or (Cii) increasing doses of purified mouse MPO. Dotted line represents unstimulated DCs. (Ciii) Representative flow cytometry plots showing LPS-induced DC CCR7 expression in the absence or presence of mouse MPO (0.0625 μg/mL). (D) DQ-OVA uptake/processing (flow cytometry) by BM-derived murine DCs cultured (2 hours) with DQ-OVA (which exhibits green fluorescence upon proteolytic degradation) and LPS/H2O2 in the presence or absence of mouse MPO (0.125 μg/mL). (E) WT (n = 6) and Mpo−/− mice (n = 6) were injected subcutaneously with DQ-OVA (50 μg) and LPS (30 μg). Uptake/processing of DQ-OVA by DCs was assessed in draining LNs 4 hours later (flow cytometry). Data are representative of 2-3 independent experiments. Each group in (C-D) contained 4 replicates. *P < .05, **P < .01, ***P < .001.
MPO suppresses DC Ag uptake/processing and migration to LNs. The frequency of DCs (CD11chi cells; flow cytometry) in draining LNs of WT (n = 8) and Mpo−/− mice (n = 8) 18 hours after subcutaneous OVA/LPS (Ai) or OVA/CFA (Aii) injection or (Aiii) OVA/LPS-injected WT mice receiving DMSO (n = 8) or ABAH (n = 8). (B) BM-derived CD45.1+ DCs (+OVA/LPS) were injected subcutaneously into CD45.2+ WT (n = 6) and Mpo−/− recipients (n = 6). Accumulation of the transferred DCs in draining LNs was assessed 18 hours later (flow cytometry). (C) CCR7 expression (flow cytometry) on BM-derived murine DCs cultured (18 hours) with LPS (1 μg/mL), H2O2 (100 μM) and (Ci) with or without supernatant (5% v/v) from CB/fMLP-degranulated WT or Mpo−/− neutrophils or (Cii) increasing doses of purified mouse MPO. Dotted line represents unstimulated DCs. (Ciii) Representative flow cytometry plots showing LPS-induced DC CCR7 expression in the absence or presence of mouse MPO (0.0625 μg/mL). (D) DQ-OVA uptake/processing (flow cytometry) by BM-derived murine DCs cultured (2 hours) with DQ-OVA (which exhibits green fluorescence upon proteolytic degradation) and LPS/H2O2 in the presence or absence of mouse MPO (0.125 μg/mL). (E) WT (n = 6) and Mpo−/− mice (n = 6) were injected subcutaneously with DQ-OVA (50 μg) and LPS (30 μg). Uptake/processing of DQ-OVA by DCs was assessed in draining LNs 4 hours later (flow cytometry). Data are representative of 2-3 independent experiments. Each group in (C-D) contained 4 replicates. *P < .05, **P < .01, ***P < .001.
MPO may suppress DC accumulation in LNs by reducing their survival and/or migration. DC apoptosis in LNs was not affected by MPO deficiency (supplemental Table 7). However, increased numbers of transferred DCs migrated to LNs of Mpo−/− mice (Figure 6B). In vitro, WT, but not Mpo−/−, neutrophil supernatant decreased LPS-induced CCR7 up-regulation by DCs (Figure 6Ci). In addition, purified mouse MPO dose-dependently reduced DC CCR7 expression (Figure 6Cii-iii).
MPO suppresses DC Ag uptake/processing
Culture of DCs with DQ-OVA (which exhibits green fluorescence upon proteolytic degradation) showed that MPO suppressed DQ-OVA uptake/processing by DCs in vitro (Figure 6Di-ii). In vivo, MPO deficiency increased DQ-OVA uptake/processing by DCs in LNs (Figure 6Ei-ii).
MPO from human neutrophils inhibits the activation of human DCs
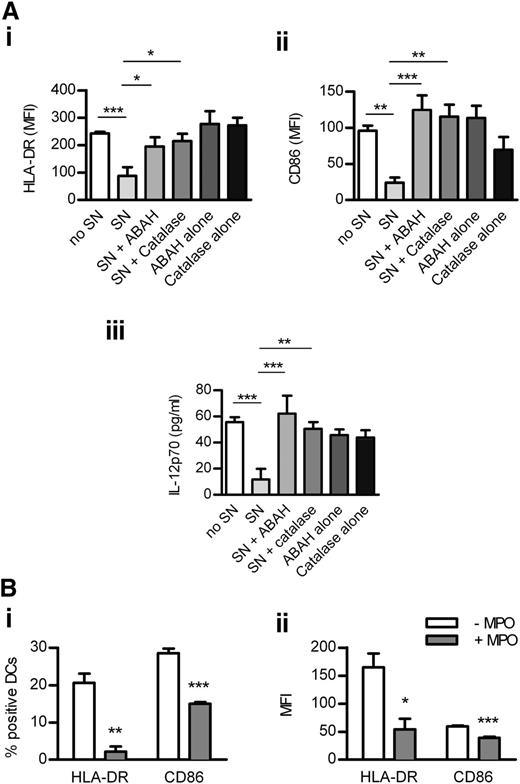
Human neutrophils were degranulated in the presence or absence of ABAH or catalase (which inhibits MPO indirectly by consuming H2O2),26 both of which reduced MPO activity in supernatant from GM-CSF/fMLP−stimulated neutrophils (supplemental Figure 6). MPO-containing supernatant down-regulated LPS-induced activation of monocyte-derived DCs, an effect reversed by inhibiting MPO with ABAH or catalase (Figure 7A). DC activation was not affected by ABAH or catalase alone (Figure 7A). In addition, purified enzymatically active (supplemental Figure 6) human MPO decreased the activation of human DCs (Figure 7B). These effects of MPO were not caused by increased DC death (not shown).
MPO from human neutrophils reduces the activation of monocyte-derived DCs. Monocyte-derived DCs were cultured (24 hours) with LPS (1 μg/mL), H2O2 (100 μM), and (A) with or without SN (60% v/v) from human neutrophils degranulated with GM-CSF/fMLP in the presence or absence of ABAH or catalase or (B) with or without purified enzymatically active human MPO (5 μg/mL). Control wells in panel A contained DCs cultured with LPS/H2O2 and ABAH or catalase alone. DC activation was assessed by measuring the expression of HLA-DR and CD86 (flow cytometry) and production of IL-12 (ELISA). Data are representative of 3 independent experiments. All groups contained 4 replicates. *P < .05, **P < .01, ***P < .001. SN, supernatant.
MPO from human neutrophils reduces the activation of monocyte-derived DCs. Monocyte-derived DCs were cultured (24 hours) with LPS (1 μg/mL), H2O2 (100 μM), and (A) with or without SN (60% v/v) from human neutrophils degranulated with GM-CSF/fMLP in the presence or absence of ABAH or catalase or (B) with or without purified enzymatically active human MPO (5 μg/mL). Control wells in panel A contained DCs cultured with LPS/H2O2 and ABAH or catalase alone. DC activation was assessed by measuring the expression of HLA-DR and CD86 (flow cytometry) and production of IL-12 (ELISA). Data are representative of 3 independent experiments. All groups contained 4 replicates. *P < .05, **P < .01, ***P < .001. SN, supernatant.
Discussion
MPO, the major neutrophil protein, is implicated as a local mediator of inflammatory injury in many chronic human diseases, making it an important therapeutic target.1,2 The current studies demonstrate that MPO diminishes the induction of adaptive immunity by inhibiting DCs to attenuate tissue inflammation. These results add to the complexity of the roles played by MPO in health and disease, detracting from the simplistic view that MPO acts solely as an injurious molecule in inflammatory conditions. Our data also support the growing recognition of a complex interaction between innate and adaptive immunity, providing new insights into how LN-infiltrating neutrophils shape the adaptive immune response and the mechanisms by which neutrophil products limit DC maturation and consequent induction of injurious T-cell responses. They define MPO as a regulator of DC activation to prevent the generation of overexuberant T-cell immunity.
Here, enhanced generation of T-cell responses attributable to MPO deletion, supported by observations in experimental glomerulonephritis and MS,5,27 resulted in exacerbated AIA, a T-cell−driven model of rheumatoid arthritis,28 indicating that any potential benefit of MPO absence in the joint was overridden by enhanced arthritogenic immunity in secondary lymphoid organs. Augmentation of joint inflammation in Mpo−/− mice was supported by increased skin DTH, demonstrating that MPO-mediated suppressive effects on the induction of T-cell responses cause less tissue inflammation.
We hypothesized that MPO suppresses adaptive immunity by inhibiting DC function because neutrophils, the major MPO source, interact with and affect DCs.8,-10 Here, consistent with previous reports,10,11,13 neutrophils rapidly and transiently infiltrated draining LNs after OVA/LPS injection. Although it is possible that some of these cells are immature granulocytic myeloid-derived suppressor cells (because they are also CD11b+Ly-6G+)29 which may, therefore, contribute to MPO-mediated effects on DCs, they displayed standard features of mature neutrophils, including high Ly-6G expression and granularity (side-scatterhi). Confocal microscopy demonstrated the presence of intracellular and extracellular MPO in LNs at the peak of neutrophil infiltration (4 hours after Ag injection), with studies of neutrophil CD68 surface expression and apoptosis suggesting that early MPO deposition in LNs by neutrophils occurs mainly through primary granule release. Importantly, extracellular MPO made contact with DCs in LNs, suggesting its potential to affect DC function. MPO may also interact with DCs in the skin, since neutrophil-DC contacts occur at inflammatory sites.7 The majority of extracellular MPO in LNs is believed to come from the infiltrating neutrophils rather than resident macrophages because (i) neutrophils are the major cellular source of MPO containing about 5 times more MPO than monocytes, and (ii) MPO expression is generally lost during monocyte-to-macrophage differentiation.1,2 H2O2, an essential cofactor for extracellular MPO-catalyzed formation of reactive intermediates, is most likely generated by neutrophil NAD phosphate (NADPH) oxidase and released by the same MPO-depositing neutrophils in LNs.1 Some H2O2 may come from other myeloid cells within LNs capable of producing H2O2 such as macrophages and, to a lesser extent (due to lower NADPH oxidase expression), DCs.30
Culture of DCs with WT/Mpo−/− neutrophil supernatant (±ABAH) or purified mouse MPO (±ABAH) showed that MPO inhibits DC activation in vitro via its catalytic activity, effects that were not attributed to MPO-mediated consumption of H2O2. Partial reversal of MPO-mediated DC inhibition by ABAH or Mpo−/− supernatant at greater doses of neutrophil supernatant used is most likely the result of the presence of other neutrophil enzymes such as elastase, which is released upon CB/fMLP stimulation and can suppress DC activation.19,31 Studies in Mpo−/− mice demonstrated that MPO also inhibits DC maturation in LNs after immunization but not as the result of MPO deficiency in DCs themselves because DCs do not express MPO.32 In vivo MPO inhibition not only confirmed the results in Mpo−/− mice but demonstrated, consistent with our in vitro data, that MPO suppresses DC activation and subsequent T-cell responses in vivo by its enzymatic activity. Notably, by transferring peptide-loaded DCs cultured with WT/Mpo−/− supernatant or MPO±ABAH, we confirmed that MPO suppresses Ag-specific adaptive immunity by inhibiting DCs.
Importantly, the suppressive enzymatically dependent effects of MPO on DC activation were demonstrated in a human system by culturing monocyte-derived DCs with supernatant from human neutrophils degranulated in the presence or absence of ABAH or with enzymatically active purified human MPO. Here, we further confirmed that the MPO H2O2-dependent enzymatic activity is required for its inhibitory effects on DCs because MPO could not reduce DC activation in the presence of catalase, which consumes H2O2.26 These results, together with our data in mice and observational studies in humans showing a greater incidence of chronic inflammatory diseases, including arthritis in patients with MPO deficiency,33,34 lower blood leukocyte MPO activity in MS and diabetes patients,35,36 and increased risk of developing lupus nephritis in patients with decreased expression of MPO due to a genetic MPO polymorphism,37 suggest that MPO also limits DC activation and consequent generation of injurious adaptive immunity in humans.
We further demonstrated that HOCl and HOBr, formed by MPO catalysis of Cl and Br,2 are the major oxidants by which MPO suppresses DC activation in vitro. This finding is supported by previous studies in which authors showed that HOCl and HOBr, both powerful oxidants, can affect cell function.38 HOSCN, generated by MPO-catalysis of SCN,39 had a minor role in DC inhibition, consistent with HOSCN being a less reactive intermediate.39 HOCl, HOBr and/or HOCl can affect protein function1,2,40 and signaling by NFκB and mitogen-activated protein kinase41,42 in other cells. Therefore, they may down-regulate DC maturation by modulating signaling pathways and/or surface receptors involved in DC activation.
MPO can also consume NO,2 which itself can attenuate DC activation.43 Here, DC CD86 expression was NO-independent. However, exogenous NO itself decreased DC IL-12 production, as reported,43 and MPO-mediated suppression of IL-12 expression in the presence of Cl alone was completely lost when SNP was also present. This finding suggests that, in vitro, the inhibitory effects of MPO-HOCl on DC IL-12 production are counter-balanced by MPO-mediated consumption of NO. These results, together with our animal data showing increased DC costimulatory molecule expression and IL-12 production due to MPO deletion or inhibition, suggest that, in vivo, MPO-generated intermediates (HOCl, HOBr, and/or HOSCN) have a dominant suppressive role in DC activation over any potential MPO-NO interactions.
Ligation/activation of DC Mac-1 by natural ligands or anti-Mac-1 antibodies inhibits DC maturation,25,44 and MPO affects Mac-1 signaling in other cells.45 Here, DC CD86 expression was Mac-1 independent, as reported.25 MPO may decrease DC CD86 expression through other pathways such as mitogen-activated protein kinase.46 In contrast, ligation/activation of Mac-1 decreased DC IL-12 production in the absence of MPO as reported,25 and abolished MPO-mediated inhibition of IL-12 expression. This suggests an interaction between the MPO-HOCl and Mac-1 pathways in DC IL-12 production. The nature of this interaction is unknown, but because Mac-1 can be activated not only by ligand binding, but also by free radicals,47 it is possible that MPO-derived oxidants activate DC Mac-1 (an effect which is bypassed by antibody-mediated Mac-1 activation) thus reducing IL-12 production.
In addition to DC maturation, full induction of T-cell responses requires DC Ag uptake/processing and migration to LNs. DQ-OVA studies showed that MPO regulates Ag uptake/processing by DCs. Although the mechanisms for this are unknown, it is possible that MPO-HOCl inactivate the DC mannose receptor (which is involved in OVA uptake) and/or proteins involved in Ag digestion.48,49 We also showed that MPO limits DC migration to LNs after immunization by reducing their CCR7 expression. Because MPO oxidants can inactivate chemotactic factors,50 neutrophil deposition of MPO in LNs may also inactivate LN DC-attracting chemokines.
In summary, we have demonstrated that MPO deposited by neutrophils in LNs interacts with DCs in vivo during the generation of adaptive immunity. MPO, via enzymatically generated reactive intermediates, attenuates adaptive immunity by suppressing murine (and human) DC activation, Ag uptake/processing and migration to LNs, resulting in decreased pathological tissue inflammation. These findings, supported by observational studies in patients with genetic deficiency or decreased expression of MPO,33,,,-37 provide cautionary information when considering MPO as a therapeutic target.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alice Wright, Lynelle Jones, and Leon Moussa for technical assistance, and James Ngui, Paul Hutchinson, and Kim Steegh for help with flow cytometry.
Supported by grants from the National Health Medical Research Council of Australia.
Authorship
Contribution: D.O. designed the research, performed experiments, collected and analyzed data, and wrote the paper; A.R.K., E.F.M., and S.R.H. designed the research and analyzed data; and Y.Y., K.M.O., R.C.M.M., K.L.E., D.S.Y.T., and S.A.S. performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen R. Holdsworth, Department of Medicine, Monash University, Monash Medical Centre, Level 5 Block E, 246 Clayton Rd, Clayton, Victoria 3168, Australia; e-mail: stephen.holdsworth@monash.edu.

![Figure 1. MPO deficiency enhances T-cell responses leading to increased skin DTH and AIA. (A-F) WT (n = 8) and Mpo−/− mice (n = 8) were immunized subcutaneously with OVA/CFA and CD4+ T-cell activation (A) and proliferation (B) assessed in LNs and spleen 6 days later (flow cytometry). (C) Concentrations of cytokines in 72-hour supernatants from OVA-stimulated splenocytes (ELISA). (D-E) Intracellular staining for IFN-γ and IL-17A in OVA-stimulated CD4-labeled splenocytes. Spleen cells were cultured (48 hours) in the presence of OVA (10 μg/mL) with brefeldin-A (5 μg/mL) added for the last 8 hours. CD4-labeled cells were then stained intracellularly for IFN-γ and IL-17A. The percentage of CD4+ T cells producing IFN-γ and IL-17A (Di), and IFN-γ and IL-17A expression by CD4+ T-cell (expressed as mean fluorescence intensity; Dii) are shown. (E) Representative flow cytometry plots showing IFN-γ (Ei) and IL-17A (Eii) production by CD4+ T cells from WT and Mpo−/− mice. (F) Skin DTH, expressed as the difference in thickness of footpads injected with OVA (right footpad) or BSA (left footpad). (G) CD4-enriched CFSE-labeled naïve OT-II T cells were transferred intravenously to WT (n = 4) and Mpo−/− recipient mice (n = 4) on d0. Recipient mice were injected subcutaneously with OVA/CFA on d2. (Gi) Proliferation of OT-II T cells in draining LNs of recipient mice 72 hours after OVA/CFA immunization (based on CFSE dilution; flow cytometry). (Gii-iii) IFN-γ production by OT-II T cells in recipient mice (intracellular staining of CD4/Vβ5-labeled splenocytes cultured with OVA323-339 [1 μM]). (Hi) The development of mBSA-induced AIA in WT (n = 8) and Mpo−/− (n = 9) mice. (Hii) Representative photomicrographs of formalin-fixed, paraffin-embedded 3-μm-thick Safranin-O-stained (fast green/hematoxylin counterstain) joint sections of WT and Mpo−/− mice with mBSA-AIA showing more severe disease in MPO-deficient animals. Sections were examined on a Leica BMLB Laboratory microscope with a ×5/0.70 NA objective lens. Images were captured with a Leica DC 300F digital camera using Photoshop CS software (Adobe Systems). Original magnification, ×50. Data are representative of 2-3 independent experiments. *P < .05, **P < .01, ***P < .001, •P = .07, #P = .1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/20/10.1182_blood-2012-09-456483/2/m_4195f1.jpeg?Expires=1766078970&Signature=jLZ79Zah2r~6LhSS9SoRlYL1slgcCn1AqdU65CIu2TpDqC77jGsPb5GE-FLFK7K8-y~p2NUrMXXRreqK7JtnpJnNV4~Khdx7GoDf0l95h~MawUGzM2Z~5qo62QyszcIu3IzPCnxr6fd-9VZeukKI1ygGVo8hN~njDCQUZy7DzDteYNR0eeUs5DBg3TssTHFDZ7GklhEo9CyXvP39lnC37MgP7tqRbxa-0AskQ5IQQlZt3x~U3zAv5F4HbGUhTYHVHKNO7zFwuNMoK9dolt6xKWbsW8oll7OgJ4bgjdl7LJ6W8pZFwnD7h8KSNHcHE9HryGMd6TzUp0ScHKWFJBn9GA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. MPO deficiency enhances T-cell responses leading to increased skin DTH and AIA. (A-F) WT (n = 8) and Mpo−/− mice (n = 8) were immunized subcutaneously with OVA/CFA and CD4+ T-cell activation (A) and proliferation (B) assessed in LNs and spleen 6 days later (flow cytometry). (C) Concentrations of cytokines in 72-hour supernatants from OVA-stimulated splenocytes (ELISA). (D-E) Intracellular staining for IFN-γ and IL-17A in OVA-stimulated CD4-labeled splenocytes. Spleen cells were cultured (48 hours) in the presence of OVA (10 μg/mL) with brefeldin-A (5 μg/mL) added for the last 8 hours. CD4-labeled cells were then stained intracellularly for IFN-γ and IL-17A. The percentage of CD4+ T cells producing IFN-γ and IL-17A (Di), and IFN-γ and IL-17A expression by CD4+ T-cell (expressed as mean fluorescence intensity; Dii) are shown. (E) Representative flow cytometry plots showing IFN-γ (Ei) and IL-17A (Eii) production by CD4+ T cells from WT and Mpo−/− mice. (F) Skin DTH, expressed as the difference in thickness of footpads injected with OVA (right footpad) or BSA (left footpad). (G) CD4-enriched CFSE-labeled naïve OT-II T cells were transferred intravenously to WT (n = 4) and Mpo−/− recipient mice (n = 4) on d0. Recipient mice were injected subcutaneously with OVA/CFA on d2. (Gi) Proliferation of OT-II T cells in draining LNs of recipient mice 72 hours after OVA/CFA immunization (based on CFSE dilution; flow cytometry). (Gii-iii) IFN-γ production by OT-II T cells in recipient mice (intracellular staining of CD4/Vβ5-labeled splenocytes cultured with OVA323-339 [1 μM]). (Hi) The development of mBSA-induced AIA in WT (n = 8) and Mpo−/− (n = 9) mice. (Hii) Representative photomicrographs of formalin-fixed, paraffin-embedded 3-μm-thick Safranin-O-stained (fast green/hematoxylin counterstain) joint sections of WT and Mpo−/− mice with mBSA-AIA showing more severe disease in MPO-deficient animals. Sections were examined on a Leica BMLB Laboratory microscope with a ×5/0.70 NA objective lens. Images were captured with a Leica DC 300F digital camera using Photoshop CS software (Adobe Systems). Original magnification, ×50. Data are representative of 2-3 independent experiments. *P < .05, **P < .01, ***P < .001, •P = .07, #P = .1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/20/10.1182_blood-2012-09-456483/2/m_4195f1.jpeg?Expires=1766211004&Signature=M5Xu5fwdCSboKizW1wOCcGvWtQOWiArFKohe0cU~fmqFOxY1r6DjyJ0STFIANNcCDOJ8vVQX1aidx8F9LVY6mkhj6VMg24T5HJlEJeWs8yGfDEAmDxEpcsV8vp1gFd-EA9oGn1wUwAQxfYaIpodA67-5TOssZHV~HGbFvfWMG26EFRABUXZVIrmSq~KkI6SiK8OhHfMerSZQKQYpreZSnqR9NLAcOeC4lTAb8kSnH~p~NWMW~ZFrrXcwhcrQKMyRVEPHXGbKF6CU0toW94GrO6Mzo0wABe4Xq9VEHj9YbzyanxvtePFOYvZnCJtiYKS-S8z9SKWOghYlNlWY7E6WQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)