Key Points
Nongenomic role for IκB kinase in platelet secretion: IKK phosphorylates SNAP-23, which affects granule-plasma membrane fusion.
Pharmacologic inhibition or deletion of platelet IKK affects bleeding times.
Abstract
Platelet secretion plays a key role in thrombosis, thus the platelet secretory machinery offers a unique target to modulate hemostasis. We report the regulation of platelet secretion via phosphorylation of SNAP-23 at Ser95. Phosphorylation of this t-soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) occurs upon activation of known elements of the platelet signaling cascades (ie, phospholipase C, [Ca2+]i, protein kinase C) and requires IκB kinase (IKK)-β. Other elements of the nuclear factor κB/IκB cascade (ie, IKK-α,-β,-γ/NEMO and CARMA/MALT1/Bcl10 complex) are present in anucleate platelets and IκB is phosphorylated upon activation, suggesting that this pathway is active in platelets and implying a nongenomic role for IKK. Inhibition of IKK-β, either pharmacologically (with BMS-345541, BAY11-7082, or TPCA-1) or by genetic manipulation (platelet factor 4 Cre:IKK-βflox/flox), blocked SNAP-23 phosphorylation, platelet secretion, and SNARE complex formation; but, had no effect on platelet morphology or other metrics of platelet activation. Consistently, SNAP-23 phosphorylation enhanced membrane fusion of SNARE-containing proteoliposomes. In vivo studies with IKK inhibitors or platelet-specific IKK-β knockout mice showed that blocking IKK-β activity significantly prolonged tail bleeding times, suggesting that currently available IKK inhibitors may affect hemostasis.
Introduction
Several hundred molecules are released from activated platelets1-5 ; these components are stored in at least 3 classes of platelet granules. The small molecules (ie, adenosine 5′-diphosphate, polyphosphate, serotonin) in dense granules are important for hemostasis, given the bleeding associated with dense granule biogenesis defects (ie, Hermansky-Pudlak Syndrome).6,7 Release of polypeptides from α-granules has a more heterogeneous impact. Patients lacking α-granules (ie, Gray Platelet Syndrome) have diverse bleeding phenotypes, ranging from severe to mild.8,9 α-Granule cargo contribute to the sequelae of thrombosis (eg, tissue repair, angiogenesis, and inflammation).10 The role of lysosome release remains unclear. Secreting these components at a vascular damage site allows platelets to control the microenvironment at the lesion. Given this central role, understanding platelet secretion should identify potential therapeutic targets for modulating spurious thrombosis and ameliorating platelet-based bleeding disorders.
Exocytosis is mediated by membrane proteins called Soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNAREs). In platelets, vesicle-associated membrane protein (VAMP)-8/endobrevin (a vesicle, or v-SNARE) is required for each granule type.11 Two target membrane SNAREs (t-SNAREs) are present: the syntaxin class (syntaxin-11 being functionally relevant12 ) and the SNAP-23/25 class (SNAP-23 being functionally relevant13-15 ). The SNAREs form a trans-bilayer complex, which facilitates fusion of the granule and plasma membranes for cargo release.11,16 Although SNAREs are essential for fusion, how, when, and where they assemble into fusigenic complexes represent key secretion control points. Thus, SNARE complex assembly is determined by numerous SNARE regulators (ie, Munc18s and Munc13s) and post-translational modifications (ie, acylation and phosphorylation).17
Reports of SNARE phosphorylation focus on t-SNAREs and an array of kinases (ie, protein kinase A [PKA], IκB kinase [IKK], casein kinase II,18 GSK3β,19 and protein kinase C [PKC] isoforms). In platelets, PKC phosphorylates SNAP-23 and syntaxin-4-modulating SNARE complex formation.20 In neurons, SNAP-25 phosphorylation on Ser187 by PKC promotes syntaxin-1 binding.21 Hepp et al22 demonstrated phosphorylation of SNAP-23, on Ser95 and Ser120, in mast cells and in thrombin-activated platelets. In mast cells, SNAP-23 is phosphorylated by IKK, which is important for secretion.23 Conversely, PKA phosphorylation of SNAP-25 or syntaxin disrupts interactions.24 These reports illustrate the varied modes by which kinases control SNAREs and thus secretion.
Liu et al25 showed that IκB is phosphorylated in activated platelets; whereas, Malaver et al26 showed that inhibiting IKK affects platelet function. These data suggest a role for IKK in platelets, but a mechanistic understanding is lacking. In this article, we asked whether IKK regulates SNAP-23, and thus secretion from platelets and whether IKK could be targeted for anti-thrombotic therapeutics. We show that IKK phosphorylates SNAP-23 in response to a number of agonists and that phosphorylation is important for SNARE complex formation and membrane fusion. We further demonstrate that IKK is part of the activation cascades in platelets and that targeting IKK affects hemostasis in vivo. Our data demonstrate that IKK inhibitors may be worth exploring as anti-thrombotic agents, in addition to their anti-inflammatory and anti-cancer properties.
Methods
Antibodies and reagents
The anti-endobrevin/VAMP-8 antibody27 and anti-cellubrevin/VAMP-3, anti-syntaxin-2, -syntaxin-4, and -SNAP-23 antibodies were as described in Schraw et al.28 The anti-SNAP-23 phosphorylation-site-specific antibody was previously characterized.22 Due to the amino acids bordering the phosphorylation site (Ala98 in human SNAP-23 vs Asn98 in rodent SNAP-23), this antibody does not detect human SNAP-23. This antibody does show limited cross-reactivity with unphosphorylated SNAP-23 when used at higher concentrations. Anti-IκBα and anti-phospho-IκBα (phospho-Ser32) were from Cell Signaling Technology (Beverly, MA). The anti-phospho-Ser/Thr/Tyr antibody was from Assay Designs (Ann Arbor, MI). Appropriate secondary antibodies coupled to alkaline phosphatase were obtained from Sigma-Aldrich (St. Louis, MO). Thapsigargin, BAPTA/AM, PP2, piceatannol, RO-31-8220, Gö6976, and A23187 were from Calbiochem (San Diego, CA). Apyrase, phorbol 12-myristate 13 acetate (PMA), genistein, prostaglandin I2 (PGI2), BAY 11-7082, BMS-345541, TPCA-1, and wedelolactone were from Sigma-Aldrich Rottlerin was from Biomol (Plymouth, PA). Purified IKK was from Invitrogen (Carlsbad, CA) and PKCα was from SignalChem (Richmond, BC). Type I collagen and thrombin were from Chrono-log (Havertown, PA). U73122 and U73343 were from Alexis Biochemicals (San Diego, CA). Other reagents were of analytical grade.
Mice and genotyping
Platelet factor 4 (PF4)-Cre+ mice29 were from Dr. Radek Skoda (University Hospital Basel, Switzerland) and were genotyped as described in Ye et al.12 IKK-βflox/flox mice30 were from Dr. Manolis Pasparakis (University of Cologne, Germany) and genotyped by PCR using primers: 65 (sense): 5′-GTTCAGAGGTTCAGTCCATTATC-3′, 45 (antisense): 5′-TAGCCTGCAAGAGACAATACG-3′ and 49 (antisense) 5′-TCCTCTCCTCGTCATCCTTCG-3′ with products: WT (primers 65-45): 436 bp, IKK-β flox (primers 65-45): 533 bp, and IKK-β deletion (primers 65-49): 652 bp. Platelet-specific IKK-β knockout mice were generated by crossing PF4-Cre+ and IKK-βflox/flox mice.
Preparation of platelets
Mouse blood was collected from a ventricle and the citrated (0.38%) blood was mixed with phosphate-buffered saline, pH 7.4, and was incubated with PGI2 (10 ng/mL; 5 minutes), followed by centrifugation at 237 × g for 10 minutes at room temperature (RT). Platelet-rich plasma (PRP) was recovered and platelets were pelleted at 483 × g for 10 minutes at RT. The pellets were resuspended in HEPES/Tyrode buffer (HT; 20 mM HEPES/KOH, pH 6.5, 128 mM NaCl, 2.8 mM KCl, 1 mM MgCl2, 0.4 mM NaH2PO4, 12 mM NaHCO3, 5 mM d-glucose) supplemented with 1 mM EGTA, 0.37 U/mL apyrase, and 10 ng/mL PGI2. Platelets were washed and resuspended in HT (pH 7.4) without EGTA, apyrase, or PGI2. Platelets were counted with a Z2 Coulter Particle Analyzer (Beckman/Coulter, Fullerton, CA) and adjusted to the indicated concentrations.
Washed human platelets were prepared as described in Karim et al.31 PRP was isolated in the presence of apyrase (0.37 U/mL) and PGI2 (10 ng/mL) by centrifugation at 150 × g for 10 minutes at RT. PRP was centrifuged at 900 × g for 10 minutes and platelets were resuspended in HT containing 1 mM EGTA, apyrase, and PGI2. Platelets were washed and resuspended in HT (pH 7.4) without EGTA, apyrase, or PGI2.
Measurement of platelet granule cargo release
Platelets were labeled with 0.4 µ Ci/mL [3H]5-HT (serotonin; Perkin-Elmer, Waltham, MA) for 1 hour at RT. After washing, the platelets were resuspended in HT (pH 7.4) and CaCl2 (0.7 mM final) prior to stimulation with thrombin (0.05 U/mL; Chrono-log) for the indicated times. Hirudin (0.1 U/mL; Sigma-Aldrich) was added to stop the reaction. Platelets were incubated with BMS-345541 (5 μM) or TPCA-1 (0.5 μM) prior to stimulation. The samples were separated by centrifugation at 13 800 × g for 1 minute, the supernatants were recovered, and the pellets were lysed with 1% Triton X-100 in phosphate-buffered saline. Equal volumes of both fractions were assayed for [3H]5-HT (serotonin) for dense granules, PF4 for α-granules, and β-hexosaminidase for lysosomes as described in Schraw et al.28,32
Preparation of SNARE-containing proteoliposomes
All lipids were from Avanti Polar Lipids (Alabaster, AL). Reconstitution of v-SNARE and t-SNARE vesicles was as described in Tucker et al.33 v-SNAREs were reconstituted using a mix of 27% 1-palmitoyl-2-oleoyl-phosphatidylethanolamine, 55% 1-palmitoyl, 2-oleoyl phosphatidylcholine, 15% 1,2-dioleoyl phosphatidylserine, 1.5% N-(7-Nitro-2-1,3-Benzoxadiazol-4-yl) (NBD)-1,2-dipalmitoyl phosphatidylethanolamine (NBD-PE, donor), and 1.5% N-(lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl phosphatidylethanolamine (Rhodamine-PE, acceptor). t-SNAREs were reconstituted in 30% palmitoyl-2-oleoyl-phosphatidylethanolamine, 55% phosphatidylcholine and 15% phosphatidylserine v-SNARE (VAMP-8) and t-SNARE (SNAP-23 + syntaxin-2) vesicles were reconstituted to give ∼60 copies and ∼95 copies per vesicle, respectively. Syntaxin-2 was a surrogate for syntaxin-11, as we cannot produce recombinant syntaxin-11 with the appropriate acylation.
For fusion assays, 10 μL t-SNARE vesicles were incubated with 0.5 mM ATP, 10 mM MgCl2, and increasing IKK (0-2.0 μg/reaction) at RT. v-SNARE vesicles were incubated separately at RT. After 60 minutes, v-SNARE and half of the t-SNARE vesicles were mixed in 25 mM HEPES pH 7.4, 100 mM KCl, 1 mM dithiothreitol and fusion was monitored at 37°C. Calcium (1 mM final) was added at t = 20 minutes. The increase in NBD fluorescence was measured using a Bio-TEK FLx800 Microplate Fluorescence Reader (Bio-Tek U.S., Winooski, VT) and KC4 software with data acquisition every 1.5 minutes. After 60 minutes, 15 μL of 5% n-dodecyl-β-d-octylglucoside was added to obtain the maximum fluorescence. Fusion was plotted as the percent of maximum fluorescence over time. Some aliquot of t-SNAREs were analyzed by immunoblotting using anti-phospho-Ser95 and anti-SNAP-23 antibodies. For inhibitor studies, 5 μM BMS-345541 or 0.5 μM TPCA-1 were added to the t-SNARE mixture containing 1.0 μg IKK and fusion was monitored after 60 minutes.
Immunoblotting
Platelet proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon-P PVDF membranes (Millipore Corp., Bedford, MA). They were then probed with the indicated primary antibodies and visualized with an appropriate alkaline phosphatase-coupled secondary antibody using enhanced chemifluorescent substrate (GE Healthcare, Piscataway, NJ). Images were obtained with a Typhoon 9400 Variable Mode Imager and quantified with ImageQuant 5.2 software (GE Healthcare, Piscataway, NJ).
Immunoprecipitation
Immunoprecipitation was carried out as described in Karim et al.34 Platelets were incubated in the presence or absence of inhibitor and activated with thrombin (0.1 U/mL) followed by lysis with 2× lysis buffer (40 mM Tris-HCl [pH 7.5], 150 mM NaCl, 16 mM Benzamidine, 2 mM Na2EDTA, 2 mM EGTA, 2% Triton X-100, 2% sodium deoxycholate, 5 mM sodium pyrophosphate, 2 mM Na3VO4, phosphatase inhibitor cocktail, and protease inhibitor cocktail). This buffer differs from our previous studies.12 The lysates were clarified by centrifugation and the supernatants were incubated with rabbit IgG and Protein A-Sepharose 4 Fast Flow (GE Healthcare, Piscataway, NJ). SNARE complexes were immunoprecipitated with anti-syntaxin-2, -4, or -11 antibodies and Protein A-Sepharose (GE Healthcare). The immunoprecipitates were analyzed by SDS-PAGE and immunoblotting.
Measurement of protein phosphorylation using [32P]-labeled platelets
Platelets, in phosphate-free HT (pH 6.5), were incubated with [32P] H3PO4 (2 mCi/mL) for 90 min at RT, and suspended in HT (pH 7.4). The labeled platelets were preincubated with inhibitors and then stimulated with thrombin. The reactions were stopped with SDS-PAGE sample buffer and the labeled proteins were separated by SDS-PAGE and visualized by phosphorimaging using the Typhoon 9400 Imager.
Bleeding time measurements
BMS-345541 was administered by gavage to C57Bl6/J male mice (18-22 g). BMS-345541 was formulated as a 2 mg/mL stock in 3% Tween 80.35 Ten mg/kg of drug per mouse was administered 2 hours prior to the measurement of bleeding time. The mice were sedated with ketamine/xylazine and their tails were transected 3 mm from the tip. The tail was placed in saline at 37°C and the time to blood flow cessation was measured. Clotting was not considered complete until bleeding had stopped for 1 minute. When required, measurements were terminated at 10 minutes. PF4 Cre+:IKK-βflox/flox and littermate control, PF4 Cre−:IKK-βflox/flox mice, at ∼6 weeks of age, were analyzed using the same method. All studies were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Results
SNAP-23 is phosphorylated upon platelet activation by IKK
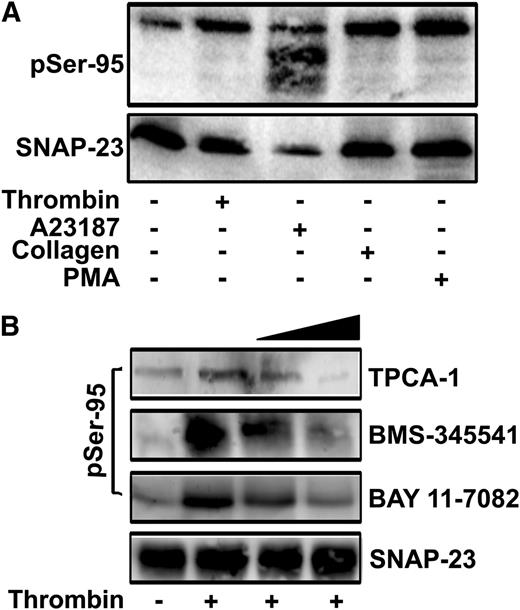
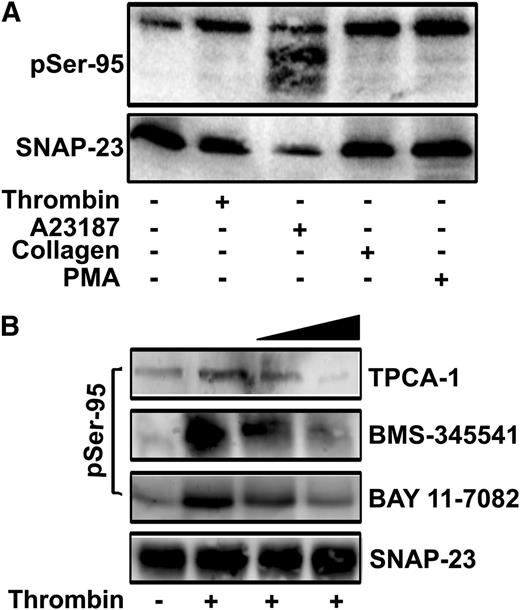
Previously, we showed that SNAP-23 is phosphorylated on Ser95 and Ser120 in thrombin-stimulated mouse platelets.22 Here, we examined Ser95 phosphorylation in response to other agonists. The phospho-Ser95-specific antibody detected phospho-SNAP-23 in platelets stimulated with thrombin, A23187, PMA, and collagen (Figure 1A). The residual phospho-SNAP-23 in resting platelets could reflect some degree of activation during preparation or could result from a limited cross-reactivity of the antibody to unphosphorylated forms of SNAP-23. The degradation of SNAP-23 and phospho-SNAP-23 in A23187-stimulated platelets is consistent with reports that calpain cleaves SNAP-23 in highly activated platelets.36,37 μ-Calpain cleaves between Gly164 and Asn165 (supplemental Figure 1) but Ser95 phosphorylation does not affect cleavage. These data confirm that SNAP-23 is phosphorylated on Ser95 and show that SNAP-23 phosphorylation occurs in response to a range of agonists.
SNAP-23 phosphorylation in stimulated platelets. (A) Mouse platelets were stimulated with the indicated agonists (thrombin, 0.1 U/mL; A23187, 1 μM; collagen, 10 μg/mL; PMA, 200 nM) for 3 minutes and were subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (B) Mouse platelets were preincubated with increasing concentrations of IKK inhibitors (TPCA-1, 0.5, 1 μM; BMS-345541, 5, 10 μM; BAY 11-7082, 12.5, 25 μM) for 5 minutes, stimulated with thrombin (0.1 U/mL; 3 min), and extracts from them were subjected to immunoblotting with pSer-95 and anti-SNAP-23 antibodies.
SNAP-23 phosphorylation in stimulated platelets. (A) Mouse platelets were stimulated with the indicated agonists (thrombin, 0.1 U/mL; A23187, 1 μM; collagen, 10 μg/mL; PMA, 200 nM) for 3 minutes and were subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (B) Mouse platelets were preincubated with increasing concentrations of IKK inhibitors (TPCA-1, 0.5, 1 μM; BMS-345541, 5, 10 μM; BAY 11-7082, 12.5, 25 μM) for 5 minutes, stimulated with thrombin (0.1 U/mL; 3 min), and extracts from them were subjected to immunoblotting with pSer-95 and anti-SNAP-23 antibodies.
SNAP-23 phosphorylation contributes to mast cell exocytosis and is mediated by IKK in these nucleated cells.22,23 To assess whether IKK is responsible for SNAP-23 phosphorylation in platelets, mouse platelets were incubated with four IKK inhibitors (BAY 11-7082, BMS-345541, TPCA-1, and wedelolactone), stimulated, and the extracts were probed for Ser95 phosphorylation. Treatment with BMS-345541, TPCA-1, or BAY 11-7082 inhibited thrombin-stimulated SNAP-23 phosphorylation in a dose-dependent manner (Figure 1B). Wedelolactone blocked phosphorylation but also affected the agonist-dependent, intra-platelet calcium transients (data not shown), suggesting an off-target effect. None of the remaining IKK inhibitors grossly affected platelet morphology (as measured by EM) or platelet activation, as measured by Ca2+ influx or global tyrosine phosphorylation (supplemental Figures 2 and 3) and Malaver et al.26
Previous studies showed that IκB is phosphorylated on Ser32 upon platelet activation, suggesting that IKK is activated in stimulated platelets.25,26 Consistently, we detected the 3 subunits of IKK (α, β, and γ/NEMO) as well as an upstream activator, the CARMA1, Bcl10, Malt-1 (CBM) complex, in both human and mouse platelets (Figure 2A). IκB phosphorylation occurred upon platelet activation with thrombin and was blocked by BMS-345541, TPCA-1, and BAY 11-7082 (Figure 2B-C).
IKK enzyme is active and the upstream signaling CBM complex is present in platelets. (A) Mouse and human platelets were held resting (R) or stimulated with thrombin (0.1 U/mL, 3 minutes; S) and extracts from them were subjected to immunoblotting with anti-CARMA1, anti-MALT1, anti-Bcl10, anti-IKK-α, anti-IKK-β, and anti-IKK-γ/NEMO antibodies. (B-C) Mouse platelets were preincubated with IKK inhibitors (BMS-345541, 5 μM; TPCA-1, 0.5 μM [B]) or BAY 11-7082, 12.5 μM; [C]) for 5 minutes and stimulated with thrombin prior to immunoblotting the extracts with anti-pSer32-IκB (pIκB) or anti-IκB antibodies.
IKK enzyme is active and the upstream signaling CBM complex is present in platelets. (A) Mouse and human platelets were held resting (R) or stimulated with thrombin (0.1 U/mL, 3 minutes; S) and extracts from them were subjected to immunoblotting with anti-CARMA1, anti-MALT1, anti-Bcl10, anti-IKK-α, anti-IKK-β, and anti-IKK-γ/NEMO antibodies. (B-C) Mouse platelets were preincubated with IKK inhibitors (BMS-345541, 5 μM; TPCA-1, 0.5 μM [B]) or BAY 11-7082, 12.5 μM; [C]) for 5 minutes and stimulated with thrombin prior to immunoblotting the extracts with anti-pSer32-IκB (pIκB) or anti-IκB antibodies.
IKK is important for platelet secretion
SNAP-23 phosphorylation temporally correlated with dense granule release38 and BAY 11-7082 treatment altered ATP release and P-selectin exposure in activated platelets.26 These data indirectly implicate IKK in platelet secretion. To directly address this, platelets were treated with BMS-345541 (5 μM) (Figure 3A) or TPCA-1 (0.5 μM) (supplemental Figure 4) stimulated with thrombin (0.05 U/mL) and released from dense granules, α-granules, and lysosomes was measured. Untreated platelets showed time-dependent release of cargo from all 3 granules. Both IKK-β inhibitors blocked secretion by >70%. These data show that inhibition of IKK-β, using conditions that blocked SNAP-23 phosphorylation, affected release from dense granules, α-granules, and lysosomes.
IKK activity is important for platelet secretion. (A) Mouse platelets were prepared as described in our “Methods” section. After adding 0.7 mM CaCl2, platelets were incubated with (open symbols) or without (●) 5 μM BMS-345541 for 3 minutes, then stimulated with thrombin (0.05 U/mL) for the indicated times. (B) IKKβ−/− platelets (○) and platelets from Cre− littermate controls (●) were stimulated with thrombin (0.05 U/mL) under similar conditions. Release of [3H]5-HT from dense granules (Dense), PF4 from α-granules (Alpha), and β-hexosaminidase from lysosomes (Lysosome) was measured and percent secretion was calculated. Each data point represents the mean of triplicates and the standard deviation is indicated. (C) IKK-β+/+ and IKK-β−/− platelets were subjected to immunoblotting with anti-IKK-β antibody. (D) IKK-β+/+ and IKK-β−/− platelets were stimulated with 0.1 U/mL thrombin for 3 minutes and subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies.
IKK activity is important for platelet secretion. (A) Mouse platelets were prepared as described in our “Methods” section. After adding 0.7 mM CaCl2, platelets were incubated with (open symbols) or without (●) 5 μM BMS-345541 for 3 minutes, then stimulated with thrombin (0.05 U/mL) for the indicated times. (B) IKKβ−/− platelets (○) and platelets from Cre− littermate controls (●) were stimulated with thrombin (0.05 U/mL) under similar conditions. Release of [3H]5-HT from dense granules (Dense), PF4 from α-granules (Alpha), and β-hexosaminidase from lysosomes (Lysosome) was measured and percent secretion was calculated. Each data point represents the mean of triplicates and the standard deviation is indicated. (C) IKK-β+/+ and IKK-β−/− platelets were subjected to immunoblotting with anti-IKK-β antibody. (D) IKK-β+/+ and IKK-β−/− platelets were stimulated with 0.1 U/mL thrombin for 3 minutes and subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies.
To confirm the role of IKK-β, we generated a lineage-specific, IKK-β-deletion mouse strain by crossing IKK-βflox/fox mice with a strain containing a PF4 promoter-driven Cre recombinase transgene.29 Platelets from these mice had no detectable IKK-β (Figure 3C) and SNAP-23 phosphorylation at Ser95 was undetectable in thrombin-stimulated platelets (Figure 3D). As with the inhibitor-treated platelets, release from each class of granules was inhibited in IKK-β−/− platelets (Figure 3B). Platelet counts and granule cargo levels were unaffected (data not shown) and there was no overt effect on platelet morphology (supplemental Figure 2B), indicating that loss of IKK-β had no effect on granule or platelet biogenesis. Further examination using lumi-aggregometry showed that release of ATP from dense granules in response to thrombin (0.05, 0.1 U/mL), collagen (10, 20 μg/mL), convulxin (0.1 μg/mL), PMA (200 nM) or A23187 (1 μM) was negatively affected by the loss of IKK-β. The effect on aggregation in response to these agonists was more variable but was generally attenuated (supplemental Figure 5).
Phosphorylation of SNAP-23 affects SNARE complex formation and membrane fusion
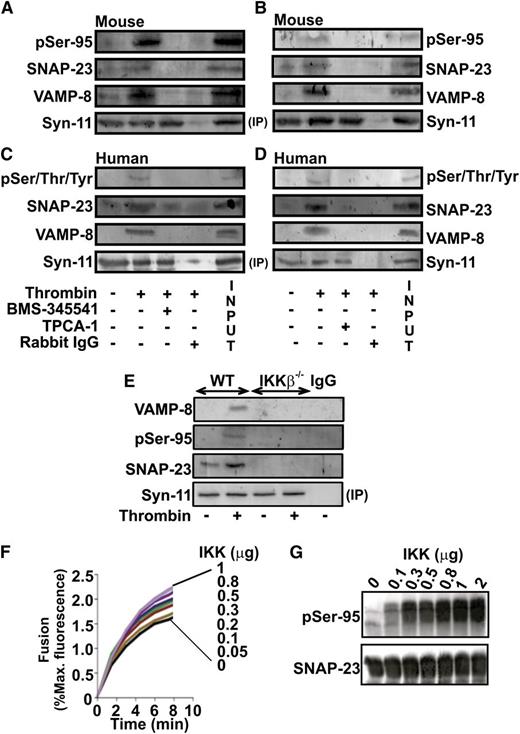
Ye et al12 demonstrated that syntaxin-11, together with SNAP-23 and VAMP-8, are important for platelet exocytosis. Previous studies showed that syntaxin-4 and VAMP-2 preferentially bind to phospho-SNAP-23 during mast cell degranulation.22,23 Therefore, we sought to determine if IKK phosphorylation of SNAP-23 contributed to SNARE complex formation in activated platelets. SNARE complex formation was monitored in drug-treated (BMS-345541 or TPCA-1) mouse (Figure 4A-B) or human (Figure 4C-D) platelets by co-immunoprecipitation with syntaxin-11 antibodies. The anti-phospho-Ser95 antibody only recognizes mouse SNAP-23,22 thus, human SNAP-23 phosphorylation was assessed using an anti-pSer/Thr/Tyr antibody. In untreated platelets, there was an activation-dependent increase in SNARE complex as evident by the presence of VAMP-8 in the immunoprecipitate. In both human and mouse platelets, the IKK-β inhibitors (BMS-345541, Figure 4A-C; TPCA-1, Figure 4B-D) not only blocked phosphorylation of SNAP-23 but also affected SNAP-23 and VAMP-8 association with syntaxin-11, indicative of an effect on SNARE complex formation. Virtually identical results were obtained when IKK-β−/− platelets were examined (Figure 4E). Platelets also contain syntaxin-2 and -4, which form SNARE complexes upon platelet activation.39 Immunoprecipitation with anti-syntaxin-2 or -4 antibodies co-precipitated phospho-SNAP-23, as well as VAMP-3 and VAMP-8 (supplemental Figure 6A-D). Both IKK-β inhibitors blocked formation of these complexes. These data demonstrate that phosphorylation of SNAP-23 by IKK affects SNARE complex formation.
IKK-mediated phosphorylation of SNAP-23 is crucial for SNARE complex formation. Mouse (A-B) and human (C-D) platelets were incubated with IKK inhibitors (BMS-345541, 5 μM) (A-C); (TPCA-1, 0.5 μM) (B-D) and stimulated with thrombin (0.1 U/mL, 3 minutes). Wild-type (WT) and IKK-β−/− mouse platelets were stimulated with thrombin (0.1 U/mL) (E). Platelet lysates were precleared and then incubated with anti-syntaxin-11. Immunoprecipitates were separated by SDS-PAGE and immunoblotted using antibodies to syntaxin-11, SNAP-23, phosho-Ser/Thr/Tyr (pSer/Thr/Tyr), phospho-Ser95 (pSer-95), and VAMP-8 as indicated. (F) SNARE-bearing vesicles, t- and v-SNARE were reconstituted as described in our “Methods” section. t-SNARE vesicles were incubated with 0.5 mM ATP, 10 mM MgCl2, and increasing amounts of IKKβ (0-1.0 μg/reaction) at RT. After 60 minutes, v- and t-SNARE vesicles were mixed and fusion was monitored at 37°C. After 60 minutes, n-dodecyl-β-d-octylglucoside was added to obtain the maximum NBD fluorescence and fusion was calculated as the percent of that maximum. (G) An aliquot of the t-SNARE mixture from some of the reactions was subjected to immunblotting using anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. The data are representative of 2 replicates.
IKK-mediated phosphorylation of SNAP-23 is crucial for SNARE complex formation. Mouse (A-B) and human (C-D) platelets were incubated with IKK inhibitors (BMS-345541, 5 μM) (A-C); (TPCA-1, 0.5 μM) (B-D) and stimulated with thrombin (0.1 U/mL, 3 minutes). Wild-type (WT) and IKK-β−/− mouse platelets were stimulated with thrombin (0.1 U/mL) (E). Platelet lysates were precleared and then incubated with anti-syntaxin-11. Immunoprecipitates were separated by SDS-PAGE and immunoblotted using antibodies to syntaxin-11, SNAP-23, phosho-Ser/Thr/Tyr (pSer/Thr/Tyr), phospho-Ser95 (pSer-95), and VAMP-8 as indicated. (F) SNARE-bearing vesicles, t- and v-SNARE were reconstituted as described in our “Methods” section. t-SNARE vesicles were incubated with 0.5 mM ATP, 10 mM MgCl2, and increasing amounts of IKKβ (0-1.0 μg/reaction) at RT. After 60 minutes, v- and t-SNARE vesicles were mixed and fusion was monitored at 37°C. After 60 minutes, n-dodecyl-β-d-octylglucoside was added to obtain the maximum NBD fluorescence and fusion was calculated as the percent of that maximum. (G) An aliquot of the t-SNARE mixture from some of the reactions was subjected to immunblotting using anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. The data are representative of 2 replicates.
To further address the effects of SNAP-23 phosphorylation on SNARE complex formation, IKK was titrated into an in vitro fusion assay containing 2 types of proteoliposomes: VAMP-8-containing and SNAP-23/syntaxin-2-containing (syntaxin-2 is a surrogate for syntaxin-11). Fusion was monitored by fluorescence dequenching (Figure 4F). There was a dose-dependent increase in fusion rate, which correlated with phosphorylation of SNAP-23 (Figure 4F-G). Fusion did not occur when the t-SNAREs were omitted (supplemental Figure 4E). The enhancement of fusion was dependent on IKK activity and was blocked by addition of either BMS-345541 or TPCA-1 (supplemental Figure 6E-F). These data are consistent with the effects in intact platelets (Figure 3) and demonstrate that IKK can directly affect SNARE complex formation and membrane fusion.
Signaling steps required for SNAP-23 phosphorylation
Agonist-induced platelet secretion is triggered via second messengers and activated kinases. To understand the place of IKK in platelet signaling cascades, we determined if IKK-mediated SNAP-23 phosphorylation required these elements. In Figure 1, the diacylglycerol (DAG) mimetic, PMA, stimulated SNAP-23 phosphorylation. To assess the importance of intra-platelet calcium, platelets were incubated with BAPTA/AM or EGTA prior to stimulation. Alternatively, intra-platelet calcium was mobilized with thapsigargin (Figure 5A). BAPTA/AM treatment dampened SNAP-23 phosphorylation, whereas EGTA had little effect. Thapsigargin stimulated phosphorylation of Ser95. These data highlight the importance of intra-platelet calcium to SNAP-23 phosphorylation.
The role of platelet signaling cascades in IKK activation and SNAP-23 phosphorylation. (A) Mouse platelets were preincubated with BAPTA/AM (40 μM) or EGTA (1 mM) for 3 minutes and then stimulated with either thrombin (0.1 U/mL) or thapsigargin (1 μM) for 3 minutes, and subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (B) Mouse platelets were preincubated with different inhibitors: 10 μM, rottlerin; 1 μM, Gö6976; and 1 μM, RO-31-8220 and PLC inhibitor (U-73122, 5 μM) or its inactive isomer (U-73343, 5 μm) for 5 minutes and then stimulated with thrombin (0.1 U/mL, 3 minutes). Extracts from these platelets were subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (C) Mouse platelets were preincubated with the indicated PKC inhibitors then activated with thrombin (0.1 U/mL, 3 minutes) and extracts from them were subjected to immunoblotting with anti-pSer32-IκB (pIκB) or anti-IκB antibodies. (D) Mouse platelets were labeled with [32P] H3PO4 then preincubated with the indicated inhibitors (1 μM, RO-31-8220 or 5 μM BMS-345541) prior to stimulation with thrombin (0.1 U/mL). The reactions were stopped with SDS sample buffer, the total platelet proteins were separated by SDS-PAGE, and the phospho-proteins were visualized by phosphorimaging.
The role of platelet signaling cascades in IKK activation and SNAP-23 phosphorylation. (A) Mouse platelets were preincubated with BAPTA/AM (40 μM) or EGTA (1 mM) for 3 minutes and then stimulated with either thrombin (0.1 U/mL) or thapsigargin (1 μM) for 3 minutes, and subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (B) Mouse platelets were preincubated with different inhibitors: 10 μM, rottlerin; 1 μM, Gö6976; and 1 μM, RO-31-8220 and PLC inhibitor (U-73122, 5 μM) or its inactive isomer (U-73343, 5 μm) for 5 minutes and then stimulated with thrombin (0.1 U/mL, 3 minutes). Extracts from these platelets were subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (C) Mouse platelets were preincubated with the indicated PKC inhibitors then activated with thrombin (0.1 U/mL, 3 minutes) and extracts from them were subjected to immunoblotting with anti-pSer32-IκB (pIκB) or anti-IκB antibodies. (D) Mouse platelets were labeled with [32P] H3PO4 then preincubated with the indicated inhibitors (1 μM, RO-31-8220 or 5 μM BMS-345541) prior to stimulation with thrombin (0.1 U/mL). The reactions were stopped with SDS sample buffer, the total platelet proteins were separated by SDS-PAGE, and the phospho-proteins were visualized by phosphorimaging.
The role of kinases and phospholipases, known to be downstream of PAR-mediated platelet activation, were probed using selective inhibitors. Pre-treatment of platelets with the tyrosine kinase inhibitor, genistein (150 μM), a Src-family kinase inhibitor, PP2 (10 μM), or the Syk inhibitor, piceatannol (10 μM), had no effect on thrombin-stimulated SNAP-23 phosphorylation (supplemental Figure 7). Upon stimulation, phospholipase Cs (PLCs) generate IP3 and DAG, which leads to PKC activation. U73122, a general PLC inhibitor, affected SNAP-23 phosphorylation but the inactive isomer U73343 did not (Figure 5B). To assess the role of PKC, we used 3 different PKC-selective inhibitors: RO-31-8220 (1 μM, pan-PKC,), Gö6976 (1 μM, PKC α/β-selective), and rottlerin (10 μM, PKC δ-selective). All 3 inhibitors affected thrombin-induced SNAP-23 phosphorylation implying a role for PKC (Figure 5B). These data show that PLC activation, DAG formation, calcium mobilization, and PKC activation are important to SNAP-23 phosphorylation. Based on these results, it is not clear which PKC isoform plays a dominant role; this will require further investigation.
Our data, and that of others, suggest that either IKK or PKC could phosphorylate SNAP-23.20 To clarify the order in which these kinases act, we determined whether PKC inhibitors affected IKK activation or whether IKK inhibitors affected PKC activation. IκB phosphorylation, as a metric of IKK activation, was monitored in platelets treated with PKC inhibitors (RO-31-8220, Gö6976, or rottlerin) (Figure 5C). All of these inhibitors affected IκB phosphorylation, although none have been reported to affect IKK-β directly. [32P]-Labeled platelets were activated with thrombin (0.1 U/mL) in the presence of RO-31-8220 (1 μM) or BMS-345541 (5 μM) and the total phosphoproteins were analyzed by SDS-PAGE and phosphorimaging (Figure 5D). Thrombin-mediated activation induced the phosphorylation of a major protein at ∼47 kDa, which is pleckstrin, a PKC substrate.40 As expected, RO-31-8220 (1 μM), blocked pleckstrin phosphorylation, but BMS-345541 (5 μM) had no effect. Under these conditions, the PKC inhibitor affected IKK activation but the IKK-β inhibitor had no effect on PKC activation. These data suggest that IKK-β is the SNARE-proximal kinase in the agonist-induced platelet signaling cascades. Consistently, IKK, but not PKCα, phosphorylated Ser95 in vitro (supplemental Figure 8).
Inhibiting IKK-β affects hemostasis
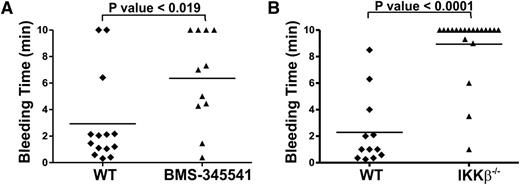
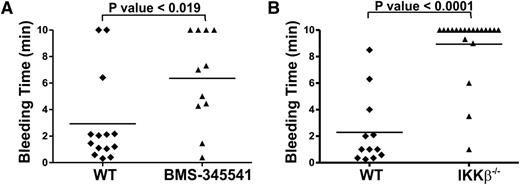
To assess the physiological importance of IKK, we inhibited it pharmacologically or deleted it genetically in platelets and measured tail bleeding times. Pharmacokinetic studies of BMS-345541 indicated that it has a limited lifetime in the bloodstream (t1/2 = 2.2 hr),35 which we considered in our study design. The compound was administered by gavage at 10 mg/kg. After 2 hours, tail bleeding time measurements were made to assess the effect of the drug on hemostasis. BMS-345541 treatment significantly prolonged bleeding times when compared with vehicle-treated, control animals (Figure 6A). Consistently, analysis of the platelet-specific, IKK-β-deletion strain yielded similar results. The fact that these mice failed to clot normally shows that platelet IKK-β is important for hemostasis (Figure 6B).
Bleeding time measurements in BMS-345541-treated and platelet-specific IKKβ−/− mice. (A) Mice were given either 3% Tween 80 (5 mL/kg, vehicle) or 10 mg/kg BMS-345541 in 3% Tween 80 by oral gavage. Two hours post-dosing, tail bleeding time measurements were made as described in our “Methods” section. (B) PF4-Cre− littermates (WT) or platelet-specific IKK-β deletion (IKK-β−/−) mice were subjected to tail bleeding measurements. Each point represents the bleeding time of a single animal. Statistical significance was determined by unpaired t test using Sigma Plot software (Systat Software, Inc., San Jose, CA).
Bleeding time measurements in BMS-345541-treated and platelet-specific IKKβ−/− mice. (A) Mice were given either 3% Tween 80 (5 mL/kg, vehicle) or 10 mg/kg BMS-345541 in 3% Tween 80 by oral gavage. Two hours post-dosing, tail bleeding time measurements were made as described in our “Methods” section. (B) PF4-Cre− littermates (WT) or platelet-specific IKK-β deletion (IKK-β−/−) mice were subjected to tail bleeding measurements. Each point represents the bleeding time of a single animal. Statistical significance was determined by unpaired t test using Sigma Plot software (Systat Software, Inc., San Jose, CA).
Discussion
Secretory granule fusion with the plasma membrane is the final step in a sequence of biochemical events that result in granule release and the modulation of the local vascular microenvironment. Because platelet secretion is pivotal to vascular health, it is critical to understand its mechanism. Here, we show that IKK is central in controlling membrane fusion. IKK, in response to platelet activation, phosphorylates SNAP-23 resulting in enhanced SNARE complex formation, enhanced membrane fusion, and granule release. Given the plethora of IKK-β inhibitors,41 our data suggest that these compounds may be useful in modulating hemostasis. Consistently, we show that platelet-specific deletion of IKK-β or treatment of mice with the IKK-β inhibitor, BMS-345541, prolonged bleeding in an in vivo model of hemostasis. Our data, along with that of Suzuki and Verma,23 clearly establishes a nongenomic role for IKK-β in platelet and mast cell exocytosis, which may be relevant in other cells types.
Activation of the NF-κB/IκB/IKK pathway in response to platelet activation has been noted by several groups.25,26,42,43 However, the role of IKK in platelets is controversial and no clear mechanism has been established. Using BAY-11-7082 and RO-106-9920, reports suggested a positive role for IKK in thrombin- or collagen-induced platelet aggregation, ATP release, TXA2 formation, and P-selectin expression.25,26 Spinelli et al43 showed that BAY-11-7082 and SC-514 affected spreading but not aggregation. Conversely, Gambaryan et al42 suggested that IKK regulated PKA by disrupting a NF-κB/IκB/PKA complex and thus acted as a negative regulator. Our data define a specific role for IKK. Using 3 different IKK-β inhibitors (BMS-345541, TPCA-1, and BAY 11-7082) and tissue-specific knockout mice, we demonstrate a positive role for IKK-β in platelet secretion. Our analyses demonstrate that the IKK-β-mediated phosphorylation of SNAP-23 augments SNARE complex formation and membrane fusion. SNAP-23 phosphorylation is probably not the sole trigger for exocytosis given the modest effects on fusion seen in Figure 4F, however, it appears to increase the efficacy of the process and represents a key step. Defective platelet secretion (especially adenosine 5′-diphosphate) negatively affects platelet activation, thus our results offer explanations for some of the data in previous reports.25,26,42,43
Isoform-selective inhibitors of PKC-α/β or -δ affected SNAP-23 phosphorylation. This is consistent with our previous report showing that Ser95 on SNAP-23 was not phosphorylated in activated PKCα−/− platelets.38 Our data imply that a PKC isoform is upstream of IKK, although it is unclear if that is true for all PKC isoforms. IKK inhibitors had no effect on phosphorylation of standard platelet, PKC substrates (ie, plekstrin) but the PKC inhibitors blocked IKK activation. This relationship between PKC isoforms and IKK has been reported in other cell types (ie, T- and B-cells) where PKC either directly or indirectly activates IKK.44 Our data suggest that this aspect of platelet activation may be similar to what is found in other hematopoietic cells types. It is also striking to note that platelets contain several toll-like receptors (TLR) and are activated by TLR ligands.45,46 Given that IKK is downstream of TLRs47 and platelets contain many of the signaling elements associated with TLR responses,45 it seems plausible that IKK activation is a signaling node responsive to both hemostatic and innate immunity stimuli.
Phosphorylation of SNAP-23 clearly promotes SNARE complex formation. In mast cells, phosphorylation affected SNAP-23 association with VAMP-2 and syntaxin-4.22,23 In platelets, Ser95 phosphorylation affects SNAP-23 association with VAMP-8 and syntaxin-11. Ser95 is conserved in both rodent and human SNAP-23. It is positioned C-terminal of the acylated cysteines that anchor SNAP-23 to membranes. In a SNARE complex, this residue is distal to the SNARE transmembrane domain and thus distal to the forming fusion pore. If the SNARE domains zipper from N- to C-terminus, the phospho-Ser95 could initiate zippering. Alternatively, this could be a spacer to separate formed SNARE complexes to orient fusion pore formation. Clearly a negative charge at that position is important because phosphomimetic mutations (Ser95-Glu/Asp) also enhance proteoliposome fusion (Zhang and Whiteheart, unpublished data). Structural studies will be required to assess the specific effects of Ser95 phosphorylation.
Finally, are IKK inhibitors potential anti-thrombotics? Burke et al35 showed that BMS-345541 is a selective inhibitor of IKK-β that affects NF-κB-dependent transcription of cytokines in vitro and in vivo. The drug was an effective anti-inflammatory agent in murine colitis.48 In vitro treatment of platelets with BMS-345541 inhibited secretion from all 3 granules. In vivo, BMS-345541 treatment increased tail bleeding times, consistent with the drug’s in vitro effects. The lineage-specific IKK-β deletion mice also showed increased bleeding times confirming the effects of the drug and suggesting that loss of platelet IKK activity accounts for the observed bleeding phenotype. To our knowledge, BMS-345541 represents the first selective IKK-β inhibitor reported to have in vivo anti-hemostatic activity. Given the number of IKK-β inhibitors that are currently available,41 our data provide important mechanistic insights to direct future uses of these drugs for cardiovascular disease.
Note in added proof
During the revision of this manuscript, Wei et al49 reported the phenotype of IKK-β deficient platelets and showed that they were secretion deficient.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mary Gail Engle and Jim Begley of the Chandler Medical Center Imaging Facility, the Kentucky Blood Center for their help; and members of the Whiteheart Laboratory for critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (HL56652 and HL082193) (S.W.W.); the American Heart Association Great Rivers Affiliate Post-Doctoral Fellowship to M.C.C.; and a grant from the National Institutes of Health Intramural Research Program (P.A.R.).
Authorship
Contribution: Z.A.K., J.Z., M.B., M.C.C., and T.R.H. performed experiments and prepared figures; R.A.H. assisted with mouse experiments; P.A.R. contributed phospho-SNAP-23-specific antibody and assisted in manuscript editing; S.W.W. analyzed data; and S.W.W. and Z.A.K prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.C.C. is Prevention Genetics, Marshfield, WI; and for T.R.H. is Vance Granville Community College, Henderson, NC.
Correspondence: Sidney W. Whiteheart, Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, Biomedical and Biological Sciences Research Building, 741 S. Limestone St, Lexington, KY 40536; e-mail: whitehe@uky.edu.

![Figure 2. IKK enzyme is active and the upstream signaling CBM complex is present in platelets. (A) Mouse and human platelets were held resting (R) or stimulated with thrombin (0.1 U/mL, 3 minutes; S) and extracts from them were subjected to immunoblotting with anti-CARMA1, anti-MALT1, anti-Bcl10, anti-IKK-α, anti-IKK-β, and anti-IKK-γ/NEMO antibodies. (B-C) Mouse platelets were preincubated with IKK inhibitors (BMS-345541, 5 μM; TPCA-1, 0.5 μM [B]) or BAY 11-7082, 12.5 μM; [C]) for 5 minutes and stimulated with thrombin prior to immunoblotting the extracts with anti-pSer32-IκB (pIκB) or anti-IκB antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-11-470468/5/m_4567f2.jpeg?Expires=1769113111&Signature=wQCI1ArjWvwxgpyYQRXgNev2HxOc47yLR955nZzYBc7UT8RJ8A83jMbC6eDW8f3y5e7TSIJJk1hGHkGmcs0tMpXfQqhx3FDh7BXVe9XMUcS0TRmxSBWPkOw7r9LNWCyesU~XQYzvilGRjKKXsvu-C2o8Y4Fw4oO9pij6AiOdnG8wO8NPaEyJGfshZ8EG4Jn5RjPhS5MwGK8q5KAN~6EaeVZCg-SvRarrkOGAAzjmhJDdVdEP8-56trs9uuiIt9a3GIpHXg6FWrpPewv83yRgnGiFGrvPyRADdoWfE2VNPcQVQGyBzQKZVvz-Mqkc2ZB906GO0yxGdAPS~JeJc00rHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. IKK activity is important for platelet secretion. (A) Mouse platelets were prepared as described in our “Methods” section. After adding 0.7 mM CaCl2, platelets were incubated with (open symbols) or without (●) 5 μM BMS-345541 for 3 minutes, then stimulated with thrombin (0.05 U/mL) for the indicated times. (B) IKKβ−/− platelets (○) and platelets from Cre− littermate controls (●) were stimulated with thrombin (0.05 U/mL) under similar conditions. Release of [3H]5-HT from dense granules (Dense), PF4 from α-granules (Alpha), and β-hexosaminidase from lysosomes (Lysosome) was measured and percent secretion was calculated. Each data point represents the mean of triplicates and the standard deviation is indicated. (C) IKK-β+/+ and IKK-β−/− platelets were subjected to immunoblotting with anti-IKK-β antibody. (D) IKK-β+/+ and IKK-β−/− platelets were stimulated with 0.1 U/mL thrombin for 3 minutes and subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-11-470468/5/m_4567f3.jpeg?Expires=1769113111&Signature=eo-hxIv5YcquW6wrPHAZ5OwlG2Hu0JLGCBG-wdblAdcF-UJIvTBOopVDFJqeDBrllhDvw21ze999Oa0V7LCVkKY0UFIgKQnOHts632tEu73jq7Un0bT8VyZqgMOEilEqMZwbGaDWPD5lXlIN-Voq50Qkiqqfn1hwiX9NvDk5xPOC9TtXl-tpDjjplQAMgRZbzDmSB-wM5ggTCSbV2Dly2q1UgXBvRBq01W7Ab80ZzPwndMkTSBOa6m98-PezRYPZWr0V66TXjVFFDpouDwjjYxgp9eVag1x0rn3mO04u34fO-4BXUNzkEXdPwXUy-qNsRjrINTibwq1Ec-ZEARqUuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. The role of platelet signaling cascades in IKK activation and SNAP-23 phosphorylation. (A) Mouse platelets were preincubated with BAPTA/AM (40 μM) or EGTA (1 mM) for 3 minutes and then stimulated with either thrombin (0.1 U/mL) or thapsigargin (1 μM) for 3 minutes, and subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (B) Mouse platelets were preincubated with different inhibitors: 10 μM, rottlerin; 1 μM, Gö6976; and 1 μM, RO-31-8220 and PLC inhibitor (U-73122, 5 μM) or its inactive isomer (U-73343, 5 μm) for 5 minutes and then stimulated with thrombin (0.1 U/mL, 3 minutes). Extracts from these platelets were subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (C) Mouse platelets were preincubated with the indicated PKC inhibitors then activated with thrombin (0.1 U/mL, 3 minutes) and extracts from them were subjected to immunoblotting with anti-pSer32-IκB (pIκB) or anti-IκB antibodies. (D) Mouse platelets were labeled with [32P] H3PO4 then preincubated with the indicated inhibitors (1 μM, RO-31-8220 or 5 μM BMS-345541) prior to stimulation with thrombin (0.1 U/mL). The reactions were stopped with SDS sample buffer, the total platelet proteins were separated by SDS-PAGE, and the phospho-proteins were visualized by phosphorimaging.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-11-470468/5/m_4567f5.jpeg?Expires=1769113111&Signature=M~FOB0lhFjdykp8LjFvU7o-L5j2ZNq1c0dyKUNuP3KsIzTzAQ7cng21~unwbtAKzCKBanS9Vnwncp3BWe0df7nHZWGd0k0Sv1jCoethqXhHz--pLhMN~IzwJlpLpLLXs7F6lBitaMeDtCgm76yRXEdNUgF29YYY4pZGQLy7lYkG97pszqJ3c5wAFYY9QGqAmLpFOM7LNtR1klGhPFkL74U-BWgsS8QQzFQwBQqMzzpda0xhBAASK1UIlFfrMtvPlSBq6wChAbxJ1vawxE3mNpZOkzt6pFzhVJlCqernxL8Fv~lbnXeiLsMHHmSKSsqArz3xGAf-N2fOkoG14IZLvNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. IKK enzyme is active and the upstream signaling CBM complex is present in platelets. (A) Mouse and human platelets were held resting (R) or stimulated with thrombin (0.1 U/mL, 3 minutes; S) and extracts from them were subjected to immunoblotting with anti-CARMA1, anti-MALT1, anti-Bcl10, anti-IKK-α, anti-IKK-β, and anti-IKK-γ/NEMO antibodies. (B-C) Mouse platelets were preincubated with IKK inhibitors (BMS-345541, 5 μM; TPCA-1, 0.5 μM [B]) or BAY 11-7082, 12.5 μM; [C]) for 5 minutes and stimulated with thrombin prior to immunoblotting the extracts with anti-pSer32-IκB (pIκB) or anti-IκB antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-11-470468/5/m_4567f2.jpeg?Expires=1769299838&Signature=0Zzs1Ksy6BWSEngrDmM2vrD0Y4Y4CZV9RXYSUzjNEW8dVCJ8o7btkSKm8~082sgz3amNNHde5kOUqwD8uyfmgaFNbweB0AiJlt8ZaNNgU5m5HONdqgjMkRcwF85EaF-Ua6xqWJjGI9iV8QA8oKXdGSymE4wme4pCYkOXccsUZcR6vPzCiGnTsfzOAT6RSi6VA3rzTfUWkZA~CFbUVFeZ6AzJCjL8jjwUrsfO5iR768ekit~L5G6fUGlrvtSIWzGBg2c3plAZcA81lvZAuw4hOGZzDZIcuhy-W-G4cZyJxkXiNO8Z0rteaOQ7azREYBPpvFsfNKkPefYq6Kb9K1iFVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. IKK activity is important for platelet secretion. (A) Mouse platelets were prepared as described in our “Methods” section. After adding 0.7 mM CaCl2, platelets were incubated with (open symbols) or without (●) 5 μM BMS-345541 for 3 minutes, then stimulated with thrombin (0.05 U/mL) for the indicated times. (B) IKKβ−/− platelets (○) and platelets from Cre− littermate controls (●) were stimulated with thrombin (0.05 U/mL) under similar conditions. Release of [3H]5-HT from dense granules (Dense), PF4 from α-granules (Alpha), and β-hexosaminidase from lysosomes (Lysosome) was measured and percent secretion was calculated. Each data point represents the mean of triplicates and the standard deviation is indicated. (C) IKK-β+/+ and IKK-β−/− platelets were subjected to immunoblotting with anti-IKK-β antibody. (D) IKK-β+/+ and IKK-β−/− platelets were stimulated with 0.1 U/mL thrombin for 3 minutes and subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-11-470468/5/m_4567f3.jpeg?Expires=1769299838&Signature=31JJLk~pwSxnR5TcS8zvO0BFQhQYYUT~P7BqOAubI30LXRBOGzkjDdWhFxntuj--lpipSWittB6zyXMfkm12Z5XHISAPYaiDNKlZCPgCBFaoAGaUX3LTcXHAEZ-iAIxoca~Jyqry0w1fV1sWUktqVsfY6X3AUyPzg--y85WRIBVeTHlqtQl-CJyKGO2cyrGkyLYg-o9s9z7h-uiBQGhO7oIN5qCgqDdDPHkT6Eaq~yrjQw-FNIeEhXakSbBtYQ~Lk896r~uaRHqhKgMphgvTAGNmTKpkNmqSaBgQ7qx7lsot3sKsycemNm8ua3tfNJdtaVEg~DLWm8nwX9RYA5MTUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. The role of platelet signaling cascades in IKK activation and SNAP-23 phosphorylation. (A) Mouse platelets were preincubated with BAPTA/AM (40 μM) or EGTA (1 mM) for 3 minutes and then stimulated with either thrombin (0.1 U/mL) or thapsigargin (1 μM) for 3 minutes, and subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (B) Mouse platelets were preincubated with different inhibitors: 10 μM, rottlerin; 1 μM, Gö6976; and 1 μM, RO-31-8220 and PLC inhibitor (U-73122, 5 μM) or its inactive isomer (U-73343, 5 μm) for 5 minutes and then stimulated with thrombin (0.1 U/mL, 3 minutes). Extracts from these platelets were subjected to immunoblotting with anti-phospho-Ser95 (pSer-95) and anti-SNAP-23 antibodies. (C) Mouse platelets were preincubated with the indicated PKC inhibitors then activated with thrombin (0.1 U/mL, 3 minutes) and extracts from them were subjected to immunoblotting with anti-pSer32-IκB (pIκB) or anti-IκB antibodies. (D) Mouse platelets were labeled with [32P] H3PO4 then preincubated with the indicated inhibitors (1 μM, RO-31-8220 or 5 μM BMS-345541) prior to stimulation with thrombin (0.1 U/mL). The reactions were stopped with SDS sample buffer, the total platelet proteins were separated by SDS-PAGE, and the phospho-proteins were visualized by phosphorimaging.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-11-470468/5/m_4567f5.jpeg?Expires=1769299838&Signature=tp5cf7OAUL7ZeGtA2BO6atczoqiQ3HqE~yMTx0R6nZ2Bv72pAZMNDAGIBAxhpnfHz6YA7hd3puR0sq1WuCGONaDjOUqY6DK2glr0p~7wmJHCm6eOkK1QzHmnyduQDHmk9UssPgOro1Boz3tm9nEvWbf0Yk1tpRIQQV8PIHHIrz3WT5XFZaoYstlO8zqi5bXEA1xxCMoFEEcjDSgewJpBo65jwMxs1s9~0Ckfm~1wN4eGV9XA4PAfIVeIoQjPVQgxtTv3oo-Gk~DqM6pfhKauuO1AzRnZU6ohoM8c5d66uhNYIEYIdKLYaqdCmxND9xCmFO8Lu9hIzmGnj4jyFjQ3Eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)