Key Points
Levels of circulating platelets determine the degree of pathology observed during arenavirus infections.
While moderate platelet drops result in deficient immune control of an LCMV infection, severe platelet-drops result in systemic hemorrhages.
Abstract
Severe arenaviral infections in humans are characterized by clinical findings common to other viral hemorrhagic fevers (VHFs), including thrombocytopenia, leukopenia, skin and internal organ hemorrhages, high viral replication, splenic necrosis, and death. Host responses, rather than direct damage by the arenaviral replication, account for most of the observed pathology, but it is not known what protective roles platelets may have in each of the manifestations. To address this issue in an animal model, we compared nondepleted (100%), partially depleted (15%), and profoundly (< 2.5%) platelet-depletedmice infected with the mouse arenavirus lymphocytic choriomeningitis virus (LCMV). Here, we describe that systemic bleedings and death were seen only in those animals receiving the stronger depletion treatment. Furthermore, we showed that the nonhemorrhagic but partially platelet-depleted mice were unable to control the viral replication because of generalized splenic necrosis, affecting innate and adaptive immune cells. These data suggest that, by their supportive roles in hemostasis, platelets may be preventing the severe pathology observed in human arenaviral infections.
Introduction
Viral hemorrhagic fevers (VHFs) are a group of distinct infectious diseases with similar clinical manifestations in humans. The acute phase of these infections is characterized by a flu-like syndrome accompanied by fever, headache, and general malaise. Severe or fulminant cases develop into hemorrhagic fevers (HFs) leading to mucocutaneous bleedings, thrombocytopenia, leukopenia, uncontrolled viral replication, internal-organ hemorrhages, immunosuppression, multiple organ dysfunction, shock, and death. Lipid-enveloped, single-stranded RNA viruses from the families Arenaviridae (Lassa [LASV], Junin, and lymphocytic choriomeningitis virus [LCMV]), Bunyaviridae (Hanta, Crimean-Congo, and Rift Valley), Filoviridae (Ebola and Marburg), and Flaviviridae (Yellow Fever and Dengue) are the best known etiologic agents of VHFs.1 Even though these viruses infect millions of individuals annually, our understanding of their pathophysiology is currently limited. Unfortunately, animal models do not fully recapitulate the clinical manifestations of infection with VHFs, and this together with the fact that most of these viruses must be studied under high biosafety containment, represents a major roadblock to enhanced understanding.2
The 2 main clinical manifestations for all severe VHF cases in humans and nonhuman primates are defects in hemostasis that leads to a hemorrhagic/shock syndrome, high viral titers, and a suboptimal immune response. Thrombocytopenia is the most dramatic alteration in hemostasis. The mechanisms underlying its development are not fully understood, but it seems to be the combined result of a maturation arrest and/or apoptosis of megakaryocytes in the bone marrow in response to high levels of type I interferons (IFNs)–α/β,3,4 and a platelet consumption process in the periphery.5 As an example of the latter, evidence of disseminated intravascular coagulation has been consistently reported in Ebola and Marburg infections.6,7 In addition, high serum viral titers are frequently associated with leukopenia and deficient immune responses. Lymphopenia in the arenavirus Lassa and Argentine HFs strongly correlates with disease severity and widespread necrosis in the splenic marginal zone and cortical and paracortical areas of the lymph nodes.8 Large numbers of lymphocytes undergoing apoptosis are seen in Ebola and Marburg infections.7,9 The sporadic severe human and nonhuman primate cases of LCMV infections resemble LASV infections, with thrombocytopenia, leukopenia, high viral titers, involvement of liver, lungs, and kidneys, and neurologic abnormalities that were overshadowed by the severity of the systemic illness.10-13
In mice, LCMV infection generates a completely different disease probably because of adaptations gained during the long virus/natural-host coevolution. LCMV is a noncytolytic virus, which indicates that any sign of acute pathology is exclusively mediated by the host response against the infection.14 When inoculated intracranially into adult mice, a fulminant meningitis develops mediated by the migration of LCMV-specific cytotoxic T lymphocytes (CTLs) into the central nervous system (CNS). Arriving CTLs release cytokines and chemokines that attract a strong myelomonocytic infiltrate that disrupts the meningeal vasculature, causing vascular leakage, seizure, and death.15 On the other hand, when the virus is inoculated subcutaneously, intraperitoneally, or intravenously, innate immune mechanisms limit the infection until the development of a CTL response that purges the infection. Lymphoid isolates (eg, clone-13), in contrast to CNS isolates (eg, Armstrong 53b) of LCMV, replicate persistently at high viral titers in multiple organs, with a deficient CTL response in adult mice.16 Extensive research has shown that specific mutations in the glycoprotein and polymerase proteins are responsible for the biologic differences observed between isolates. Specifically, a glycoprotein mutation increases its affinity for the cellular receptor α-dystroglycan (DG),17 which is highly expressed on dendritic cells (DCs), and a polymerase mutation probably increases its activity allowing a faster replication rate in vivo.18,19 In the mouse, both mutations give the lymphotropic viruses the ability to escape from their initial confinement in macrophages of the splenic marginal zone and invade the white pulp.20 Once there, the virus infects large numbers of DCs and stromal cells (FRCs),21 and rapidly reaches blood and peripheral tissues.22 The end results of these events are deficient antigen presentation by the infected DCs, destruction of infected DCs by early generated CTLs,23 high levels of antigen presentation by nonprofessional antigen presenting cells (APCs), and the expression of immunosuppressive molecules, such as IL-10,24,25 programmed death-1,26 and transforming growth factor-β.27 Together, these factors drive the CTL response into exhaustion with diminished proliferative capacity, poor cytotoxic activity, reduced cytokine production, and for certain epitopes, the complete deletion of the response.28
Iannacone et al29 recently reported that platelet-depleted mice infected with LCMV Armstrong developed a syndrome similar to severe human cases of VHFs, with mucocutaneous bleedings, vascular leakage, anemia, uncontrolled viral replication, suboptimal immune responses, and subsequent death of the animals; lethal hemorrhage was dependent on IFN-α/β receptor signaling. Furthermore, Goerge et al30 showed that mice rendered thrombocytopenic suffered localized hemorrhages at sites undergoing noninfectious inflammatory processes, and that low numbers of circulating platelets were able to prevent such inflammation-induced hemorrhages. Therefore, murine LCMV infections where the severity of the thrombocytopenia can be controlled experimentally, represent an attractive model to study the pathophysiology of VHFs. Here we describe such a model and show that preserving at least 15% of the circulating platelets prevented systemic hemorrhages and death of the mice. We also show that even in the absence of life-threatening manifestations, viral control was not achieved, and this was associated with necrotic destruction of splenic innate and adaptive immune cells. Altogether, these observations may contribute to the understanding and clinical treatment of severe VHF infections and report a previously unappreciated relationship between viral infections, platelet-mediated hemostasis, and immunosuppression.
Methods
Mice, viruses, and infections
Five- to 8-week-old female C57BL/6J mice were purchased from The Jackson Laboratory. LCMV Armstrong and clone-13 isolates were obtained from the laboratory of Dr Rafi Ahmed and triple plaque purified; viral stocks were prepared from infected (0.01 multiplicity of infection) BHK-21 cell supernatants 72 hours after infection. Titers from supernatants, serum, or homogenized tissue samples were determined by standard plaque assays on vero cells. All infections were done intravenously with 2 × 106 plaque-forming unit (PFU) of the specific virus, as indicated in “Results.” All infections were carried out under ABSL-3 confinement at Yerkes National Primate Center and performed in accordance with Emory University Institutional Animal Care and Use Committee guidelines. These studies were approved by the Emory University Institutional Animal Care and Use Committee.
Platelet depletions, platelets counts, and thrombopoietin treatment
Platelets were depleted by intraperitoneal injection into the lower-left abdominal quadrant of 0.2 mL of antibodies against GPIIb (MWReg30, BD Bioscience), GPIbα (R300, Emfret), or Aspercetin (isolated as previously described)31 diluted to concentrations indicated in “Results.” Capillary blood was collected directly from the tip of the tails of the mice into a BD Unopette system test to count platelets in a hemocytometer according to the manufacturer's instructions. Recombinant mouse thrombopoietin (TPO; R&D Systems) produced in murine myeloma cells was injected intravenously at 5 days and 1 day before infection.
Flow cytometry and live/dead discrimination
Spleens were collected from animals and, after collagenase IV digestion and lysis of red blood cells, single-cell suspensions were counted and stained with fluorochrome-conjugated antibodies obtained from BD Pharmingen, eBioscience, and Biolegend. Flow cytometric analysis was performed on LSR-II or FACSCalibur flow cytometers (BD Bioscience), and data were analyzed using FlowJo Version 9.5.2 software (TreeStar). Live/dead discrimination was performed using the amine-reactive dye Alexa Fluor 430 carboxylic acid-succinimidyl ester, using protocols adapted from the manufacturer (Invitrogen). Splenocytes from naive mice were used to set the gates at zero percent necrosis.
Histology and immunofluorescence microscopy
Tissues were collected from killed animals, formaldehyde-fixed, sectioned, and stained with hematoxylin and eosin (H&E). Spleens were removed and frozen in OCT (Tissue Tek). Cryostat sections (6 μm) were fixed in ice-cold acetone, air dried and stained with monoclonal antibodies to F4/80, B220, Thy1.2, and CD11c from eBioscience, to ER-TR9, ER-TR7, and MOMA-1 from Acris GmbH, and to β3 integrin from BD Pharmigen. Guinea pig anti-LCMV sera was used to visualize viral antigens. Images were obtained using a Zeiss Axioscope Z.1 microscope equipped with a Zeiss Imager 2.1 camera.
Peptide stimulations and tetramer staining
Intracellular staining for IFNγ and TNFα after 6 hours in vitro stimulation with 2.5μM GP33, GP276, or NP396 peptide in the presence of 1 μL/mL of brefledin A was performed using the Cytofix/Cytoperm kit according to the manufacturer's instructions (BD Pharmigen). Major histocompatibility complex (MHC) class I tetramers were prepared and used as described.32
Cytokine analysis
Measurement of IFN-α/β serum bioactivity was performed as previously described.33 RayBio mouse cytokine antibody array-1 (RayBiotech) was used as instructed by the manufacturer.
Statistical analysis
Statistical analysis was performed with an unpaired t test using GraphPad Prism Version 4.0 software.
Results
Low levels of circulating platelets prevent severe systemic hemorrhage and lethality during acute LCMV infection
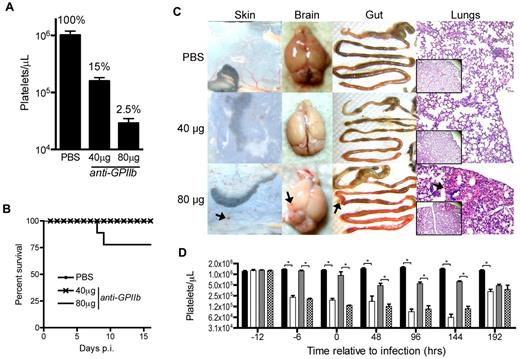
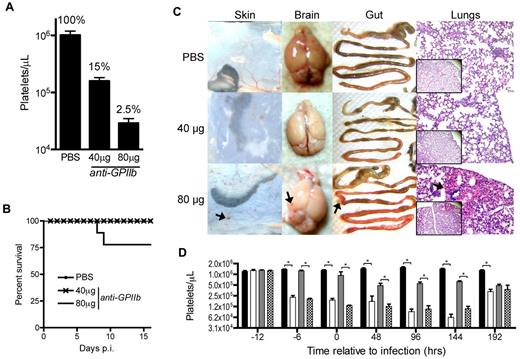
To investigate the role of platelets in the control of LCMV infections, we developed a depletion protocol that induces partial or complete depletion of platelets in mice. Zero, 40, or 80 μg of anti-GPIIb monoclonal antibody were injected intraperitoneally in the mice and 24 hours later blood was collected for platelet quantification in the absence of infection. To simplify titration of the platelet depleting reagents, we chose a single anti-GPIIb monoclonal antibody instead of the more commonly used anti-GPIbα antibody cocktail, and the intraperitoneal route for a slower diffusion and depletion rate, resulting in a more controlled study. Under these conditions we managed to preserve approximately 15%-20% of the circulating platelets when the animals received the 40-μg treatment, but saw an almost complete depletion (± 2.5%) in mice receiving the 80-μg dose (Figure 1A).
Approximately 15% of the total platelet count is sufficient to prevent lethality and peripheral organ hemorrhage during LCMV Armstrong infection. (A) Circulating platelet counts 24 hours after intraperitoneal injection of PBS, 40 μg, or 80 μg of anti-GPIIb monoclonal antibody. Groups of 5 animals per treatment, of at least 2 independent experiments. Error bars are SEM. (B) Survival of LCMV Armstrong infection after platelet depletion treatment. Mice were injected intraperitoneally with PBS, 40 μg, or 80 μg of anti-GPIIb monoclonal antibody and infected 12 hours later with 2 × 106 PFU of LCMV Armstrong intravenously. Depletion treatment was repeated 2 and 4 days after infection and survival was monitored daily for 16 days. The plot shows at least 8 animals per group from the combination of 2 experiments. (C) Signs of hemorrhage observed in infected animals treated with 80 μg of anti-GPIIb antibody. Animals treated as in panel B were killed at day 8 after infection. Skin, brains, and guts were dissected and photographed and lungs were histologically processed, H&E-stained, and micrographs were taken (100× and 40× insets). Arrows indicate hemorrhagic spots. (D) Kinetics of the thrombocytopenia induced by the anti-GPIIb treatment and the LCMV Armstrong infection (40 μg of anti-GPIIb, 12 hours before and, 2 and 4 days after LCMV Armstrong infection or PBS injection). Platelet counts were determined at the indicated time points for nontreated and noninfected mice (black bars), treated and noninfected mice (open bars), nontreated and infected mice (gray bars), and treated and infected mice (checkered bars). Error bars represent SEM (*P < .05). Data of 2 separate experiments, with 5 animals per group per experiment.
Approximately 15% of the total platelet count is sufficient to prevent lethality and peripheral organ hemorrhage during LCMV Armstrong infection. (A) Circulating platelet counts 24 hours after intraperitoneal injection of PBS, 40 μg, or 80 μg of anti-GPIIb monoclonal antibody. Groups of 5 animals per treatment, of at least 2 independent experiments. Error bars are SEM. (B) Survival of LCMV Armstrong infection after platelet depletion treatment. Mice were injected intraperitoneally with PBS, 40 μg, or 80 μg of anti-GPIIb monoclonal antibody and infected 12 hours later with 2 × 106 PFU of LCMV Armstrong intravenously. Depletion treatment was repeated 2 and 4 days after infection and survival was monitored daily for 16 days. The plot shows at least 8 animals per group from the combination of 2 experiments. (C) Signs of hemorrhage observed in infected animals treated with 80 μg of anti-GPIIb antibody. Animals treated as in panel B were killed at day 8 after infection. Skin, brains, and guts were dissected and photographed and lungs were histologically processed, H&E-stained, and micrographs were taken (100× and 40× insets). Arrows indicate hemorrhagic spots. (D) Kinetics of the thrombocytopenia induced by the anti-GPIIb treatment and the LCMV Armstrong infection (40 μg of anti-GPIIb, 12 hours before and, 2 and 4 days after LCMV Armstrong infection or PBS injection). Platelet counts were determined at the indicated time points for nontreated and noninfected mice (black bars), treated and noninfected mice (open bars), nontreated and infected mice (gray bars), and treated and infected mice (checkered bars). Error bars represent SEM (*P < .05). Data of 2 separate experiments, with 5 animals per group per experiment.
Next, we proceeded to infect mice with LCMV Armstrong 12 hours after initiating the platelet depletion. Subsequent doses of anti-GPIIb were administered at days 2 and 4 after infection and survival of the animals was monitored daily for 16 days. All animals treated with the partial depletion dose (40 μg) survived the infection until the end of the experiment, as did mice in the PBS control group. In contrast, approximately 25% of mice receiving the complete depletion treatment (80 μg) succumbed to infection around days 7 to 8 after infection (Figure 1B). The experiment was repeated with the same treatment groups, and animals were killed 8 days after infection and dissected to look for signs of hemorrhage in the skin, brain, gut, and lungs. Mice without depletion and mice with partial depletion of platelets showed no signs of hemorrhage induced by the infection in any of the tissues analyzed. On the other hand, all mice that were completely depleted of platelets had signs of systemic hemorrhage in all organs, as indicated by hemorrhagic spots (Figure 1C). Then, to better characterize the thrombocytopenic model, we analyzed the platelet kinetics of the anti-GPIIb 40-μg treatment (12 hours before infection and at day +2 and +4 after infection) in the presence or absence of infection. As shown in Figure 1D, the treatment accomplishes a significant platelet drop throughout the course of the study.
Thus, it seems that highly thrombocytopenic mice suffered severe systemic hemorrhages and were more probable to succumb to LCMV infections. This is consistent with previously published data in which mice given a stronger platelet depletion protocol (± 0.7% remaining platelets) showed massive hemorrhages and higher (± 60%) mortality.29 In contrast, it seems that a small fraction of circulating platelets were enough to completely prevent both manifestations.
Mice with reduced platelet numbers are unable to control LCMV Armstrong replication
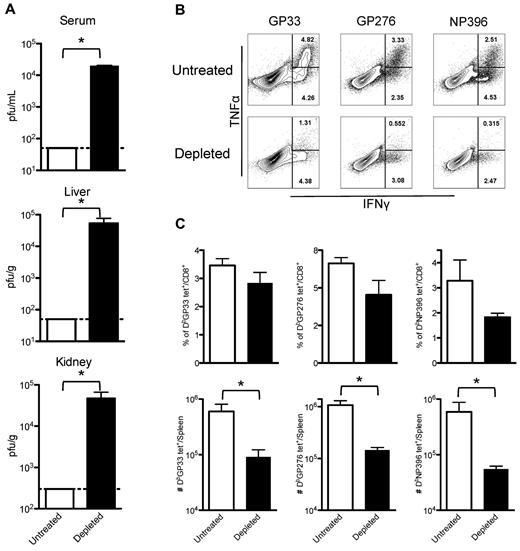
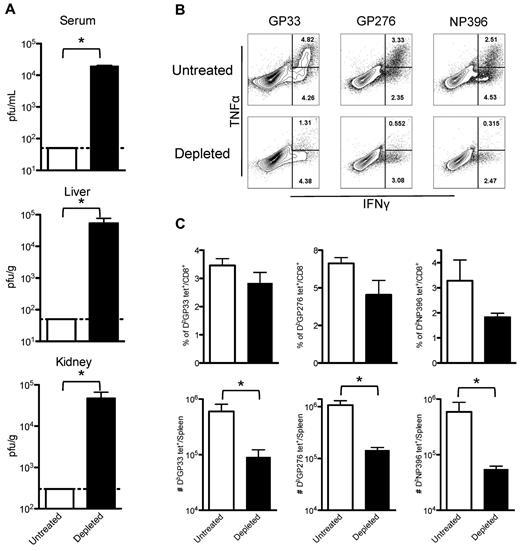
Although partial depletion of platelets followed by LCMV Armstrong infection did not lead to lethal hemorrhages, it did impair clearance of the viral infection and generated exhausted T-cell responses. Mice receiving three 40-μg doses of anti-GPIIb (at −0.5, +2, and +4 days after infection) were infected with LCMV Armstrong, and 8 days later viral titers were determined in serum, liver, and kidneys. PBS-treated animals did not have detectable virus, whereas mice with reduced levels of platelets had significant levels of virus replication in all organs examined (Figure 2A). Splenocytes recovered from these animals were restimulated in vitro with 3 different LCMV immunodominant epitopes (GP33, GP276, and NP396), and analyzed for the capacity of CD8+ T cells to produce cytokines. Figure 2B shows flow cytometry plots of gated CD8+ T cells for untreated and platelet-depleted mice. The percentage of cells capable of producing a single cytokine (IFNγ+) or double (IFNγ+, TNFα+) cytokines was severely reduced for all the epitopes tested in the infected mice that were subjected to the anti-GPIIb treatment (Figure 2B). A fraction of the same splenocytes was directly stained with fluorochrome-conjugated MHC class I tetramers specific for the same epitopes as in Figure 2B. As shown in Figure 2C, the percentage of CD8+ T cells that stained positive for each of the tetramers was reduced in the treated mice, with the NP396 population being the most affected. The defect became more significant when the total numbers of tetramer specific populations per spleen were calculated, showing an almost 10-fold reduction in each of the populations (Figure 2C). Thus, under conditions of reduced circulating platelets but in the absence of lethal hemorrhage, mice failed to clear LCMV Armstrong from serum and peripheral tissues and showed significant lower and functionally impaired LCMV-specific CD8+ T cell responses.
Partially platelet-depleted mice fail to control an acute LCMV Armstrong infection and have exhausted LCMV-specific CD8+ T-cell responses. Mice were treated with PBS or 40 μg of anti-GPIIb antibody 12 hours before and, 2 and 4 days after LCMV Armstrong infection. (A) Viral titers found in serum, liver, and kidneys. Serum and organs of infected mice were recovered 8 days after infection. (B) Cytokine production by splenic virus–specific CD8+ T cells. IFNγ and TNFα production by splenocytes 8 days after infection after in vitro stimulation with the indicated peptides (cells gated on CD8+ events, numbers indicate percentage of cells in each region). (C) Total numbers of LCMV-specific CD8+ T cells in the spleen. Day 8 after infection tetramer positive populations (as indicated in the graphs) expressed as percentage of CD8+ cells (top panel) and as total cells per spleen (bottom panel). (A-C): Open bars, PBS-treated controls; black bars, anti-GPIIb–treated mice. Error bars represent SEM (*P < .05). One representative experiment of at least 2 is shown, with 5 animals per group per experiment.
Partially platelet-depleted mice fail to control an acute LCMV Armstrong infection and have exhausted LCMV-specific CD8+ T-cell responses. Mice were treated with PBS or 40 μg of anti-GPIIb antibody 12 hours before and, 2 and 4 days after LCMV Armstrong infection. (A) Viral titers found in serum, liver, and kidneys. Serum and organs of infected mice were recovered 8 days after infection. (B) Cytokine production by splenic virus–specific CD8+ T cells. IFNγ and TNFα production by splenocytes 8 days after infection after in vitro stimulation with the indicated peptides (cells gated on CD8+ events, numbers indicate percentage of cells in each region). (C) Total numbers of LCMV-specific CD8+ T cells in the spleen. Day 8 after infection tetramer positive populations (as indicated in the graphs) expressed as percentage of CD8+ cells (top panel) and as total cells per spleen (bottom panel). (A-C): Open bars, PBS-treated controls; black bars, anti-GPIIb–treated mice. Error bars represent SEM (*P < .05). One representative experiment of at least 2 is shown, with 5 animals per group per experiment.
Leukocyte numbers and locations are not affected by platelet removal
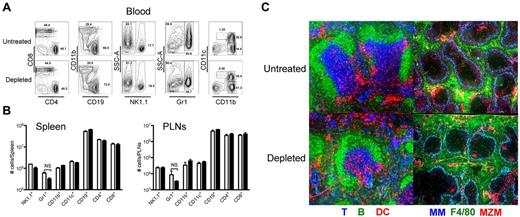
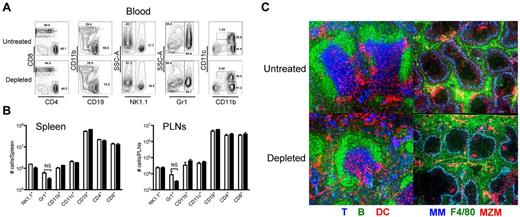
To evaluate whether the removal of platelets was affecting the blood or secondary lymphoid organ (SLO) composition and structure, we performed multicolor flow cytometry and immunohistochemistry of those tissues. Mice were killed 24 hours after injection of PBS or 40 μg of anti-GPIIb intraperitoneally, and blood, spleens, and peripheral lymph nodes (PLNs) were collected and processed as indicated in “Methods.” Flow cytometric analysis of blood leukocytes of control and platelet-depleted mice as shown in Figure 3A, showed no significant changes among the populations analyzed between the 2 groups. Single-cell suspensions of collagenase-digested SLOs were analyzed and are presented in Figure 3B as the total numbers of each individual population per organ. No difference could be detected in any of the populations analyzed in the treatment group compared with the control group. Immunostaining of splenic sections with antibodies against T, B, and DCs (left panels), or metallophillic macrophages (MM), red pulp macrophages (F4/80), and marginal zone macrophages (MZM; right panels) showed that, all the cells were properly positioned at their specific locations in the spleen. B cells were located at the B-cell follicles and marginal zones, DCs were at the bridging channels and T-cell zones, and T cells were at the T-cell zones. Likewise, all macrophage populations were present and identically distributed in the treated and untreated spleens (Figure 3C). Thus, the partial depletion of platelets with the anti-GPIIb antibody did not change the composition of the blood or SLOs, nor did it alter the splenic microarchitecture.
Platelet removal in the absence of infection does not affect the blood and SLO compartments. Mice were treated with PBS or 40 μg of anti-GPIIb antibody and killed 24 hours later. Blood, spleen, and peripheral lymphoid nodes (PLNs) were recovered and analyzed as described in “Methods.” (A) Flow plots of circulating immune cells in blood. Numbers indicate percentage of cells in each gate. (B) Number of immune cells in SLOs. Spleen and PLN cell populations after collagenase digestion, were counted, stained, and analyzed by flow cytometry. Open bars, PBS-treated controls; black bars, anti-GPIIb–treated mice. Error bars represent SEM. NS indicates nonsignificant. (C) Conserved splenic microarchitecture. Spleens were frozen in OCT medium, cut, and stained with antibodies to Thy1.2 (T cells, blue), B220 (B cells, green), and CD11c (DCs, red) or Moma-1 (metallophillic macrophages, blue), F4/80 (red pulp macrophages, green), and ER-TR9 (MZMs, red).
Platelet removal in the absence of infection does not affect the blood and SLO compartments. Mice were treated with PBS or 40 μg of anti-GPIIb antibody and killed 24 hours later. Blood, spleen, and peripheral lymphoid nodes (PLNs) were recovered and analyzed as described in “Methods.” (A) Flow plots of circulating immune cells in blood. Numbers indicate percentage of cells in each gate. (B) Number of immune cells in SLOs. Spleen and PLN cell populations after collagenase digestion, were counted, stained, and analyzed by flow cytometry. Open bars, PBS-treated controls; black bars, anti-GPIIb–treated mice. Error bars represent SEM. NS indicates nonsignificant. (C) Conserved splenic microarchitecture. Spleens were frozen in OCT medium, cut, and stained with antibodies to Thy1.2 (T cells, blue), B220 (B cells, green), and CD11c (DCs, red) or Moma-1 (metallophillic macrophages, blue), F4/80 (red pulp macrophages, green), and ER-TR9 (MZMs, red).
Early innate control of LCMV requires platelets
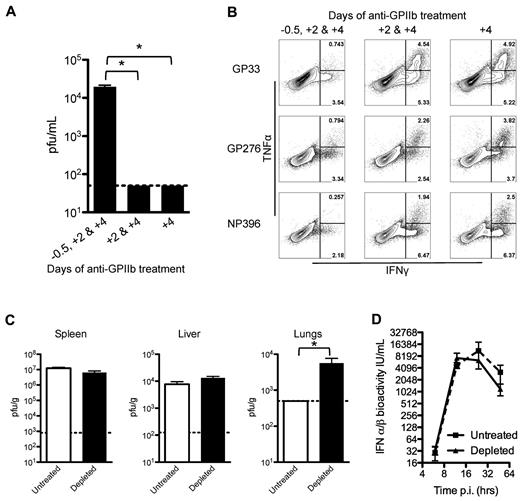
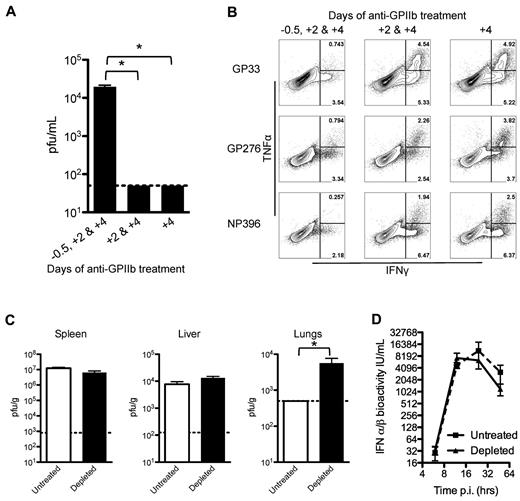
To understand the mechanisms affecting the ability to control LCMV infection by platelet removal, a kinetic analysis of the depletion was performed. Three groups of mice were treated with anti-GPIIb at different time points. The first group received 3 doses (at days −0.5, +2, and +4 after infection), the second group skipped the first dose (at days +2 and +4), and the third group received only the last dose (at day +4). When LCMV serum titers were measured at 8 days after infection, only the group that received all 3 doses of antibody had detectable levels of virus in the serum (Figure 4A). CD8+ T cells from these mice showed a marked decrease in their cytokine production only in the group that received the 3 depleting doses and had circulating virus in serum (Figure 4B). Then, we measured viral titers at 48 hours after infection in spleen, liver, and lungs, and observed that the levels of virus replication were comparable in the spleen and liver (sites of massive virus production), but viral replication in the lungs was significantly higher in the treated groups compared with undetectable levels in the control group at this early time point (Figure 4C). The previous results point to an early defect in the control of LCMV infections, which is highly associated with IFN-α/β activity. However, when the production and release of IFN-α/β was measured after infection, the removal of platelets showed no appreciable changes in serum levels of these cytokines (Figure 4D). Together, these results indicate that, under our experimental conditions, platelets need to be present at the beginning of the infection to constrain the early virus replication, by a mechanism independent of IFN-α/β production.
Early control of the virus is affected by partial platelet depletion independent of type I IFN production. Mice were treated with 3 doses (12 hours before and 2 and 4 days after infection), 2 doses (2 and 4 days after infection), or 1 dose (4 days after infection) of 40 μg of anti-GPIIb and infected with LCMV Armstrong intravenously. (A-B). (A) Platelet presence in the first 2 days of infection is needed to control the infection. Eight days after infection viral titers in serum were determined. (B) Functional exhaustion of LCMV specific CD8 T cells is restored if platelets are present in the first 2 days of infection. Intracellular IFNγ and TNFα cytokine production by day 8 splenocytes after in vitro stimulation (numbers in flow plots indicate percentage of cells inside gates). Mice were treated with 40 μg of anti-GPIIb and infected intravenously 12 hours later with LCMV Armstrong (C-D). (C) Equivalent LCMV replication in spleen and liver but higher titers in lungs 48 hours after infection in the absence of platelets. Viral titers in spleen, liver, and lung tissue homogenates. Open bars, PBS-treated controls; black bars, anti-GPIIb–treated mice. Error bars represent SEM (*P < .05). One representative experiment of at least 2 is shown. (D) LCMV-induced antiviral IFN-α/β levels. Mice were bled at 6, 12, 24, and 48 hours after infection and IFN-α/β bioactivity was determined in serum (n = 4-6 animals, in 2 independent experiments; error bars represent SEM).
Early control of the virus is affected by partial platelet depletion independent of type I IFN production. Mice were treated with 3 doses (12 hours before and 2 and 4 days after infection), 2 doses (2 and 4 days after infection), or 1 dose (4 days after infection) of 40 μg of anti-GPIIb and infected with LCMV Armstrong intravenously. (A-B). (A) Platelet presence in the first 2 days of infection is needed to control the infection. Eight days after infection viral titers in serum were determined. (B) Functional exhaustion of LCMV specific CD8 T cells is restored if platelets are present in the first 2 days of infection. Intracellular IFNγ and TNFα cytokine production by day 8 splenocytes after in vitro stimulation (numbers in flow plots indicate percentage of cells inside gates). Mice were treated with 40 μg of anti-GPIIb and infected intravenously 12 hours later with LCMV Armstrong (C-D). (C) Equivalent LCMV replication in spleen and liver but higher titers in lungs 48 hours after infection in the absence of platelets. Viral titers in spleen, liver, and lung tissue homogenates. Open bars, PBS-treated controls; black bars, anti-GPIIb–treated mice. Error bars represent SEM (*P < .05). One representative experiment of at least 2 is shown. (D) LCMV-induced antiviral IFN-α/β levels. Mice were bled at 6, 12, 24, and 48 hours after infection and IFN-α/β bioactivity was determined in serum (n = 4-6 animals, in 2 independent experiments; error bars represent SEM).
CD8 T-cell responses are affected by platelet depletion independently of high viral titers
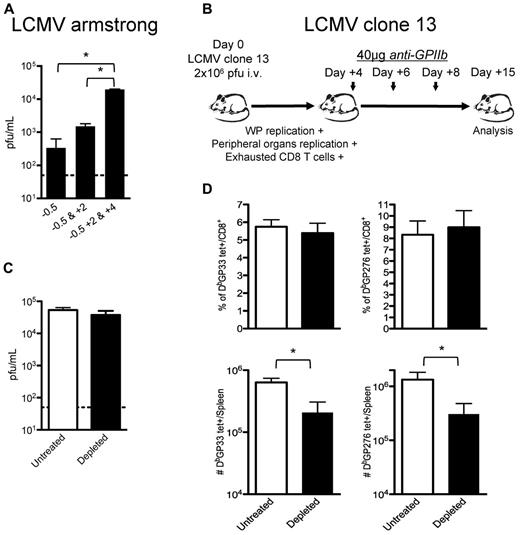
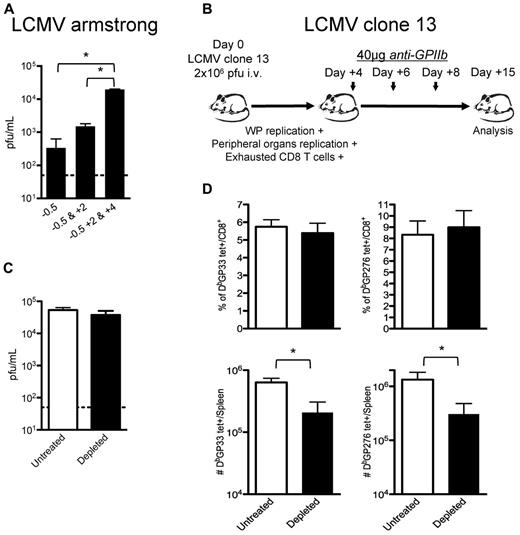
Study of the effect of platelet depletion on CD8+ T cells is complicated because of the early loss of control of the virus. The observed CD8+ T-cell exhaustion may be solely because of increased viral replication rather than a direct effect of platelet removal on these cells. However, the observation that subsequent treatments with anti-GPIIb (40 μg) enhanced the defect seen after 1 dose (Figure 5A) suggested that platelets are required for late control of LCMV Armstrong infection. To gain a better understanding of this issue, we designed a set of experiments wherein early control of the virus is lost and CD8+ T-cell responses become exhausted even in the presence of platelets. We used the LCMV clone-13 strain as an established system where the early control of the virus is deficient. Mutations in this viral strain allow the virus to infect DCs and FRCs in the splenic white pulp (WP), reach higher viral titers in peripheral tissues and blood earlier than its parental strain Armstrong, and induce the expression of immunosuppressive molecules that, altogether, contribute to the exhaustion of CD8+ T cells that fails to control the infection.18-22 Platelet removal after day 4 after infection allowed us to evaluate whether platelets were directly required in the accumulation of the CD8+ T cells independently of their exhausted status (Figure 5B). The late removal of platelets during LCMV clone-13 infections did not affect the high levels of viral replication reached 15 days after infection (Figure 5C). This may correspond to a saturation of the capacity of the mice to produce virus, as suggested by the levels reached in CD8+ deficient mice.34 No differences were observed in the exhausted CD8+ T-cell responses when we analyzed them by tetramer staining as percentages of total CD8+ T cells (top panel, Figure 5D); however, the total number of the 2 tetramer-positive populations analyzed per spleen was statistically reduced in the platelet-depleted group (bottom panel, Figure 5D), indicating that the platelet removal affected the CD8+ T-cell responses against LCMV by nonspecifically affecting the total population of CD8+ T cells. Thus, even if a defect in the innate control of the infection drives the exhaustion of the CD8+ T cells, platelet removal further affects the generation of a protective adaptive response.
CD8 T-cell responses are directly affected by partial platelet depletion independent of the levels of circulating viral antigen. (A) Continuous platelet removal enhances LCMV Armstrong replication. Mice were treated with 1 dose (12 hours before infection), 2 doses (12 hours before and 2 days after infection), or 3 doses (12 hours before and 2 and 4 days after infection) of 40 μg of anti-GPIIb and infected with LCMV Armstrong intravenously. Serum viral titers were determined at day 8 after infection. (B) Effect of platelet removal on exhausted CD8 T cells. Mice were infected with the LCMV chronic strain clone-13, which in adult mice infects the WP, reaches higher titers in blood and peripheral tissues by day 4, and generates exhausted T-cell responses (C-D). Groups of mice were infected with the chronic LCMV strain clone-13 and treated with PBS or 40μg of anti-GPIIb 4, 6, and 8 days after infection. (C) Platelet depletion of LCMV clone-13 infected animals does not affect viral replication. Viral serum titers were determined after 15 days of infection. (D) LCMV-specific CD8+ T-cell responses during the course of a chronic infection are further affected by the absence of platelets. Two different CD8+, tetramer+ populations (DbGP33 and DbGP276) were quantified on day 15 LCMV clone-13 infected splenocytes. (C-D). Open bars, PBS-treated controls; black bars, anti-GPIIb–treated mice. Error bars represent SEM (*P < .05). One representative experiment of at least 2 is shown, with 6 animals per group.
CD8 T-cell responses are directly affected by partial platelet depletion independent of the levels of circulating viral antigen. (A) Continuous platelet removal enhances LCMV Armstrong replication. Mice were treated with 1 dose (12 hours before infection), 2 doses (12 hours before and 2 days after infection), or 3 doses (12 hours before and 2 and 4 days after infection) of 40 μg of anti-GPIIb and infected with LCMV Armstrong intravenously. Serum viral titers were determined at day 8 after infection. (B) Effect of platelet removal on exhausted CD8 T cells. Mice were infected with the LCMV chronic strain clone-13, which in adult mice infects the WP, reaches higher titers in blood and peripheral tissues by day 4, and generates exhausted T-cell responses (C-D). Groups of mice were infected with the chronic LCMV strain clone-13 and treated with PBS or 40μg of anti-GPIIb 4, 6, and 8 days after infection. (C) Platelet depletion of LCMV clone-13 infected animals does not affect viral replication. Viral serum titers were determined after 15 days of infection. (D) LCMV-specific CD8+ T-cell responses during the course of a chronic infection are further affected by the absence of platelets. Two different CD8+, tetramer+ populations (DbGP33 and DbGP276) were quantified on day 15 LCMV clone-13 infected splenocytes. (C-D). Open bars, PBS-treated controls; black bars, anti-GPIIb–treated mice. Error bars represent SEM (*P < .05). One representative experiment of at least 2 is shown, with 6 animals per group.
Splenic necrosis in platelet-depleted mice affects innate and adaptive immune components
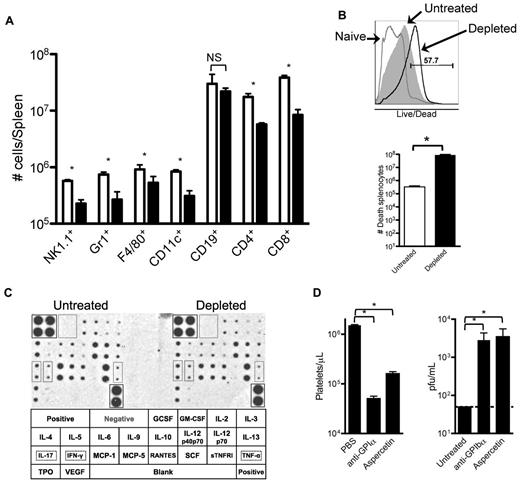
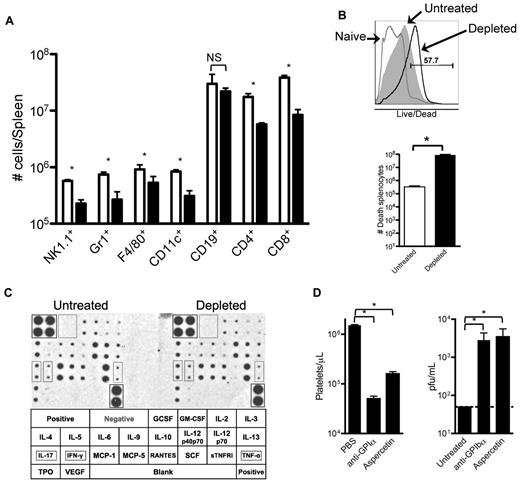
The data presented in Figures 4 and 5 indicate that removing platelets during an LCMV infection results in a generalized deficiency in elicited immune responses against the virus. To further analyze this defect, 8 days after infection with LCMV Armstrong we quantified different immune cells in the spleens of partially platelet-depleted and control mice by flow cytometry. Under partial platelet depletion, shown in Figure 6A, numbers of all innate and adaptive immune cells, with the exception of B cells, were reduced in the spleens of anti-GPIIb–treated mice, confirming our previous observations. The size of the spleens and the number of total splenocytes quantified by light microscopy were not reduced in the platelet-depleted mice (data not shown); however, most of the cells in the treated group stained positive by a live/dead flow cytometry based assay that detects disrupted cellular membranes in necrotic cells (Figure 6B). Several reasons could explain why the removal of large amount of platelets induces necrosis in the spleen. The possibility of a cytokine storm was discarded by a cytokine array performed 24 hours after infection (36 hours after platelet depletion), which showed no difference in any of the 21 cytokines analyzed (Figure 6C). Cross-reactivity of the anti-GPIIb was excluded by depleting platelets with anti-GPIbα, which recognizes a protein in a different molecular complex on the platelet (Figure 6D left panel). Finally, possible damage by deposition of large quantities of immune complexes in the spleen was ruled out by removing platelets from the blood by aggregating them in the circulation with a toxin (aspercetin; right panel, Figure 6D). Thus, it seems that the deficient immune response against LCMV infection is the result of a necrotic process that unspecifically affects the immune cells of the spleen by the reduction of functional platelets per se.
Reduced innate and adaptive immune cell populations in spleens of platelet-depleted infected animals correlates with an increase of necrotic splenocytes. Groups of mice were treated with PBS or 40 μg of anti-GPIIb antibody 12 hours before, and 2 and 4 days after LCMV Armstrong infection. (A) Splenic quantification of immune cells by flow cytometry. Collagenase-digested spleens of day 8 infected animals were quantified. (B) Detection and quantification of dead splenocytes. Flow cytometry analysis of day 8 postinfection splenocytes that were collagenase digested and stained with an in-house Alexa Fluor 430 dye protocol that detects disruptions of cell membranes. Top panel shows a representative histogram of the analysis gated on total splenocytes (gray histogram, untreated; empty histogram, depleted; numbers indicate percentage of positive cells). Bottom panel shows numbers of dead splenocytes. (C) Serum cytokine levels. Untreated and depleted animals were bled 24 hours after infection. Sera from each group were pooled and levels of 21 different circulating cytokines determined by a commercial immunoassay method with each cytokine detected in duplicate (n = 6). Bottom table shows the arrangement of the cytokines on the membranes, and 3 relevant cytokines (IL-17, IFNγ, and TNFα) were marked. (D) The absence of platelets, not the anti-GPIIb depletion protocol, is the defining factor. Mice were treated with PBS or with 2 different platelet-depleting reagents (anti-GPIbα or aspercetin), and serum viral titers were determined 8 days after infection. (A-B,D) Open bars, PBS-treated controls; black bars, platelet-depleted mice. Error bars represent SEM (*P < .05). NS indicates nonsignificant.
Reduced innate and adaptive immune cell populations in spleens of platelet-depleted infected animals correlates with an increase of necrotic splenocytes. Groups of mice were treated with PBS or 40 μg of anti-GPIIb antibody 12 hours before, and 2 and 4 days after LCMV Armstrong infection. (A) Splenic quantification of immune cells by flow cytometry. Collagenase-digested spleens of day 8 infected animals were quantified. (B) Detection and quantification of dead splenocytes. Flow cytometry analysis of day 8 postinfection splenocytes that were collagenase digested and stained with an in-house Alexa Fluor 430 dye protocol that detects disruptions of cell membranes. Top panel shows a representative histogram of the analysis gated on total splenocytes (gray histogram, untreated; empty histogram, depleted; numbers indicate percentage of positive cells). Bottom panel shows numbers of dead splenocytes. (C) Serum cytokine levels. Untreated and depleted animals were bled 24 hours after infection. Sera from each group were pooled and levels of 21 different circulating cytokines determined by a commercial immunoassay method with each cytokine detected in duplicate (n = 6). Bottom table shows the arrangement of the cytokines on the membranes, and 3 relevant cytokines (IL-17, IFNγ, and TNFα) were marked. (D) The absence of platelets, not the anti-GPIIb depletion protocol, is the defining factor. Mice were treated with PBS or with 2 different platelet-depleting reagents (anti-GPIbα or aspercetin), and serum viral titers were determined 8 days after infection. (A-B,D) Open bars, PBS-treated controls; black bars, platelet-depleted mice. Error bars represent SEM (*P < .05). NS indicates nonsignificant.
Platelet depletion during LCMV infection disrupts the splenic architecture
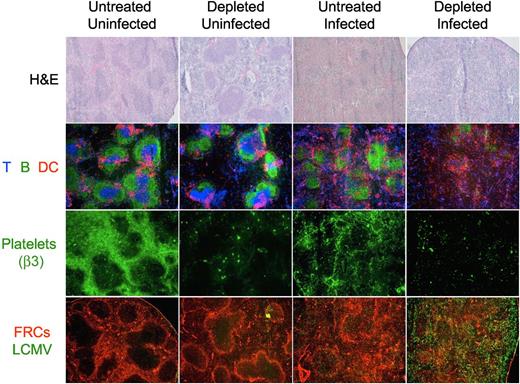
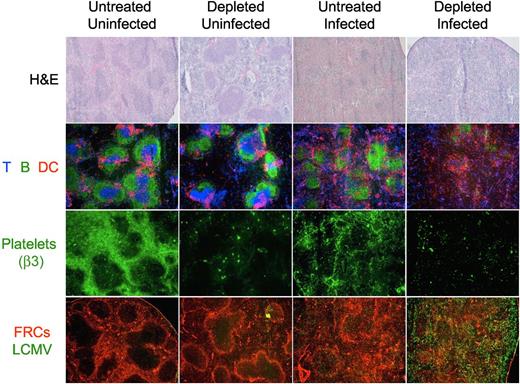
Structural analysis of the spleen of day 8 treated and untreated mice by light and immunofluorescence microscopy shows that the platelet depletion protocol during the LCMV infection induced an absolute disruption of the splenic morphology as indicated by the absence of discernable areas of white versus red pulp in the H&E staining, and the disintegration of the follicular structures by immunofluorescence. Furthermore, immunostaining of platelets with an antibody against integrin β3 (exclusively expressed on platelets) shows a reorganization of the platelets in the spleen during the LCMV infection toward structures resembling capillaries and sinusoids. Analysis of viral antigen and FRCs indicated an extensive and homogenous viral replication throughout the splenic parenchyma in the platelet-depleted mice (Figure 7).
Organization of the splenic structure is disrupted by the LCMV infection in platelet-depleted mice. Four groups of mice were treated with PBS or 40 μg of anti-GPIIb antibody 12 hours before, and 2 and 4 days after LCMV Armstrong infection or PBS injection (group 1, nontreated and noninfected; group 2, treated and noninfected; group 3, nontreated and infected; and group 4, treated and infected). Spleens were collected from mice killed 8 days later and thin tissue sections were stained for light or immunofluorescence microscopy analysis. Figure shows representative photomicrographs of H&E (40×, top row); T, B, and DCs (Thy1.2, B220, CD11c; 50×, second row); platelets (β3 integrin; 50×, third row); or fibroblastic reticular cells and LCMV (ER-TR7 and anti-LCMV, 50×, bottom row; n = 5).
Organization of the splenic structure is disrupted by the LCMV infection in platelet-depleted mice. Four groups of mice were treated with PBS or 40 μg of anti-GPIIb antibody 12 hours before, and 2 and 4 days after LCMV Armstrong infection or PBS injection (group 1, nontreated and noninfected; group 2, treated and noninfected; group 3, nontreated and infected; and group 4, treated and infected). Spleens were collected from mice killed 8 days later and thin tissue sections were stained for light or immunofluorescence microscopy analysis. Figure shows representative photomicrographs of H&E (40×, top row); T, B, and DCs (Thy1.2, B220, CD11c; 50×, second row); platelets (β3 integrin; 50×, third row); or fibroblastic reticular cells and LCMV (ER-TR7 and anti-LCMV, 50×, bottom row; n = 5).
Thrombopoietin treatment does not improve chronic LCMV viral clearance
Mice infected with the LCMV clone-13 strain suffer an acute thrombocytopenia that begins 3 to 4 days after infection and an immunosuppression that leads to the chronic replication of the virus. To determine whether there is any association between thrombocytopenia and immunosuppression in this infection, we treated mice 5 and 1 days before LCMV clone-13 infection with 5 μg (10 μg per mouse total) of TPO intravenously to increase the numbers of circulating platelets, and 15 days later we measured viral titers in serum. Even though we were able to considerably increase the numbers of circulating platelets before (approximately 2-fold) and at the nadir of the thrombocytopenia (approximately 1.6-fold at day +4), the viral titers obtained at day 15 after infection were not statistically different to the control group (data not shown); indicating that thrombocytopenia is not associated with the immunosuppression seen in the LCMV clone-13 infections.
Discussion
Here we demonstrated the importance of the physical presence of platelets in the prevention of typical clinical manifestations of severe VHFs in a murine model of LCMV infection. First we showed that systemic bleedings and death of the mice because of LCMV infection appeared only when more than 85% of platelets were depleted and second we described that even a milder removal of platelets during infection provoked a necrotic destruction of splenocytes, impairing the control of the infection. Both observations are novel and relevant to the understanding of the pathophysiology of VHFs per se, but they also highlight basic concepts on the role of platelets in supporting vascular integrity during the response to viral infections.
When mice were profoundly depleted of platelets and infected with LCMV Armstrong, hemorrhagic spots in lungs, skin, brain, and intestines were observed along with some degree of mortality. Interestingly, when the depletion treatment was reduced so that 15% of platelets in blood were present at the time of the infection, neither bleedings nor death were observed. This implies that platelet counts must drop to extremely low levels for hemorrhage and death to occur, and indirectly links the cause of death to the increased vascular leakage and systemic shock. Iannacone et al29 demonstrated that severe thrombocytopenic wild-type mice infected with LCMV developed lethal hemorrhages, that mononuclear infiltrates and viral antigens were not present at the hemorrhagic sites, and that development of hemorrhages was absolutely dependent on signaling through the IFN-α/β receptor. Furthermore, it was shown that inflammation induces hemorrhages in uninfected thrombocytopenic mice only at the specific anatomic locations were the inflammatory stimulus was applied, and that 10% to 15% of platelets were sufficient to prevent the hemorrhages.30 Altogether, these data are consistent with the hypothesis that the hemorrhages and death in severely thrombocytopenic mice infected with LCMV29 may be consequences of the high levels of IFN-α/β released into the serum, inducing a systemic inflammation that opens the vascular barriers, allowing the escape of fluids and erythrocytes into the tissues. The extreme efficiency of a small number of platelets at preventing these systemic manifestations suggests that physical adhesion and aggregation may not be required, but perhaps the mechanism in place may involve transient delivery of signals to the endothelium during platelet rolling through membrane receptors or vasoactive compounds from the platelet granules.35,36
Next, we observed that mice with reduced levels of circulating platelets, lower than normal levels but high enough to prevent hemorrhages and death, failed to control infection with LCMV Armstrong because of necrotic destruction of innate and adaptive immune splenocytes and the subsequent exhaustion of the remaining CTLs. Infection of untreated adult mice with LCMV Armstrong induces a strong and fully protective CTL response able to clear the infection in a week. If the virus is inoculated intracranially, the migration of CTLs into the CNS to fight infection provokes a fatal meningitis. Mechanistically, this is mediated by a massive myelomonocytic infiltrate recruited by the CTLs in the meninges.15 This large migration breaks the vascular barrier, allowing the release of fluids into the CNS interstitial tissues, which may ultimately be the event leading to the seizure and death of the mice. On the other hand, Ho-Tin-Noe et al35,37 showed that tumor growth is reduced in platelet-depleted mice by a tumor infiltration of innate immune cells that elicit hemorrhages resulting in the necrotic death of tumor cells. Soluble P-selectin serum levels increase during LCMV infection of mice, indicating that platelets are activated during infection.38 Altogether, we hypothesize that infection with LCMV causes a dramatic alteration of splenic structure because of the large recruitment, proliferation, and control of the infection by immune cells, generating a stressful environment for the endothelial cells. The enhanced transmigration of cells may open holes in the vascular wall, exposing extracellular matrix that activates the platelets and these subsequently adhere to prevent the leakage of fluids; in the absence of platelets large quantities of vascular fluids and erythrocytes may be released in the interstitial tissues inducing hypoxia and necrosis of splenic immune cells resembling a splenic infarction. The need for platelets to directly attach to the exposed subendothelial tissue would explain the requirement for higher numbers of platelets to prevent the splenic necrosis than systemic bleedings, and also explain the platelet activation and consumption observed during infection. In our partial platelet depletion model, loss of viral control seems to require accumulated defects of both early (innate) and late (adaptive) immune responses.
The previous was a compelling result because similar immunosuppression and uncontrolled viral replication are seen in untreated adult mice infected with the LCMV clone-13 strain. We then attempted to compensate for LCMV-induced thrombocytopenia by increasing the number of circulating platelets before infection using the megakaryocyte growth and development factor thrombopoietin, to evaluate whether the increased platelet count would improve the control of the chronic LCMV clone-13 infection in mice. Under the conditions obtained in this study, no differences were seen in the viral titers reached 15 days after infection, indicating that the immunosuppression that leads to persistent replication of this strain is not a consequence of the thrombocytopenia. Even though the number of platelets was increased significantly before and at the nadir of the thrombocytopenia versus the untreated infected mice, complete reconstitution could not be achieved, probably because of the destruction of megakaryocytes by the IFN-α/β response that induces the thrombocytopenia.3 We hypothesize that the virally-induced thrombocytopenia in LCMV clone-13 infected mice may not be severe enough to cause the immunosuppression, hemorrhage, and death; instead other mechanisms, such as altered cellular tropism and enhanced replication kinetics, account for the observed persistence of clone-13 and similar isolates in wild-type mice, the natural host.17-21 We speculate that there could be a direct link between susceptibility to severe manifestations of VHFs and platelet counts in the host before the infection, where mice are resistant (1.2 × 106/μL),39 monkeys are more susceptible (0.5 × 106/μL),40 and humans are highly affected (0.2 × 106/μL).41
The data presented in this work creates several research inquiries for future studies. First, vasoactive molecules such as S1P agonists, serotonin, and vascular endothelial growth factor could be evaluated for their ability to prevent hemorrhages and death in experimentally platelet-depleted mice. Second, our results suggest that the higher level of circulating platelets compared with other species (including humans) may explain why mice have not proven to be an adequate general experimental model for VHFs. Mice genetically deficient in TPO signaling (TPO or c-mpl knockouts), which have platelet levels similar to those found in humans and are viable, healthy, and display no signs of spontaneous bleeding, may be a better system to study its pathology.42,43 Third, we hypothesize that by selectively inactivating IFN-α/β signaling in megakaryocytes but preserving its important antiviral activity in immune and stromal cells, we would be able to completely overcome the thrombocytopenia induced by infection with LCMV clone-13 and better study if its control has any positive effect. Finally, the mechanism responsible for the splenocytes destruction should also be studied to conclude whether it is mediated by complement, oxygen/nutrient deprivation, apoptosis, or if reactive oxygen species are involved in their demise.
In conclusion, we have shown in the murine model of LCMV infection that a spectrum of clinical manifestations resembling VHFs in humans can be manipulated by careful titration of antibodies used to deplete platelets. Although nearly complete depletion of platelets produces severe hemorrhages and death, mice survive infection after partial platelet depletion but fail to control viral replication because of the necrotic destruction of innate and adaptive immune cells. Our approach opens new insights for the study of the pathophysiology of VHFs and may provide a theoretical foundation for the development of new therapies for humans.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Rafi Ahmed for providing viruses and reagents and Drs Rafi Ahmed, Allan Kirk, Aron Lukacher, Oscar Perng, Barry Rouse, and James Zimring for critical reading of the paper.
This work was supported by grants from the National Institutes of Health (1RO1AI042373), and CFAR Immunology Core grant P30 AI050409. G.D.L. was sponsored by a Fulbright scholarship. G.D.L. presented this work as a partial fulfillment to the Immunology and Molecular Pathogenesis PhD program at Emory University.
National Institutes of Health
Authorship
Contribution: G.D.L., P.A.R., and N.B.M. performed experiments and analyzed results; G.D.L. and A.R. isolated and purified special reagents; and G.D.L. and J.D.A. designed the research, made the figures, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Altman, Emory Vaccine Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30329; e-mail: jaltman@emory.edu.