Key Points
The data demonstrate the complexity of the genetic contribution to inhibitor development in people with hemophilia A.
Potentially decisive markers have been identified, indicating the importance of further evaluation of intracellular signaling pathways.
Abstract
Studies of determinants of development of inhibitory Abs to factor VIII in people with hemophilia A indicate a complex process involving multiple factors. The Hemophilia Inhibitor Genetics Study (HIGS) Combined Cohort was formed to extend our understanding of the genetic background of risk. The study group contains 833 subjects from 3 independent cohorts: brother pairs and singletons with and without a history of inhibitors, as well as 104 brother pairs discordant for inhibitor status. Using an Illumina iSelect platform, 13 331 single-nucleotide polymorphisms from 1081 genes, primarily immune response and immune modifier genes, were typed. Each cohort was analyzed separately with results combined using a meta-analytic technique. After adjustment for potential confounders, 53 single-nucleotide polymorphisms were found to be significant predictors of inhibitor status using the criteria of odds ratios in the same direction in all cohorts or allowing for a 20% interval around an odds ratio = 1 in 1 of the 3 and significant in at least 2. Of the 53 markers, 13 had meta P < .001. Eight of the 53 were significant predictors among the discordant pairs. Results support the complexity of the immune response and encourage further research with the goal of understanding the pathways involved.
Introduction
The treatment of hemophilia has improved significantly over the years, but the development of Abs that neutralize the effect of the infused factor remains a serious obstacle for patients and care givers. The reasons that only a fraction of patients, 10%-15% overall and typically 20%-30%1 among those with severe disease, develop Abs remain obscure, but there are several observations indicating that genetic factors are of major importance. The most extensively studied is the type of causative gene mutation.2 The highest risk has been associated with null mutations—those considered to result in no protein production, thereby keeping the immune system naive to the deficient factor. In particular, large deletions involving multiple domains confer high risk and yet, similar to that seen with other high-risk mutation types, there are families containing multiple siblings with this mutation who remain inhibitor free.3 Independent of the type of causative mutation, the infused factor will be endocytosed in the APCs and proteolytically degraded to smaller peptides that will be presented on the cell surface by the HLA class II molecules to the Th cells. This interaction is fundamental for the immune response to occur, and without HLA class II molecules with the ability to present the immunogenic peptides to the T cells, no immune response will take place. It is therefore not surprising that associations with HLA class II alleles such as DRB*1501 and DQB*0602 have been reported.4 A higher concordance of inhibitor status than expected between siblings and ethnic variations4,5 suggest that other genetic markers may be decisive in the determination of whether the immune response occurs. Indeed, genetic markers have been reported, independent of the type of F8 mutation, such as single nucleotide polymorphisms (SNPs) in the genes coding for IL-1, IL-2, IL-10, TNFA, TGFβ, and CTLA-4.6-12 Because several of the SNPs characterized in immunoregulatory molecules coded by the human genome will influence the level of immune modulators, immune system challenges in conjunction with replacement therapy and a genetic predisposition might interact. This is supported by the observations of brother pairs and monozygotic twins with discordant inhibitor status5 and the formation of inhibitors in association with significant inflammatory reactions among patients considered to be at low risk.13
The goal of the Hemophilia Inhibitor Genetics Study (HIGS) is to contribute to a better understanding of the genetic background of the risk for developing inhibitors. In the current analysis, a large panel of SNPs in and near immune response genes were evaluated in related and unrelated subjects with and without an inhibitor history. In addition to the HIGS cohort, 2 previously described cohorts were included in this combined analysis, the Malmö International Brother Study (MIBS)5 and the Hemophilia Growth and Development Study (HGDS).14 The advantages of combining data from these cohorts are 2-fold: (1) the larger sample size increases statistical power and (2) we are able to observe the effects of a set of genetic markers on inhibitor development in 3 studies of differing design. Our method of analysis maximizes the opportunity to identify replication of results among the cohorts while allowing for a more powerful estimation of effect size than might be derived from each individual study. The most significant findings associated with a higher or lower risk in the cohorts are described, as well as the outcome of the genetic markers in 104 brother pairs discordant for inhibitor status.
Methods
Population
The HIGS Combined Cohort includes clinical and laboratory data for 833 people with hemophilia A enrolled in 1 of 3 studies: HIGS (N = 448), MIBS (N = 120), and HGDS (N = 265). HIGS is composed of brother pairs concordant for a history of inhibitors or discordant (one with an inhibitor, the other without) and a group of singletons with inhibitors enrolled between 2004 and 2010. Participants were enrolled from Europe, North America, Latin America, and South Africa. The MIBS is composed of brother pairs with hemophilia with or without inhibitors recruited primarily from centers in Europe between 1996 and 2000. The HGDS is a population-based investigation conducted in 14 US hemophilia treatment centers. A cohort of 274 children and adolescents with hemophilia A were enrolled between 1989 and 1990. Those with DNA samples available (97%) were included in the analysis. The procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation for all 3 cohorts and with the Declaration of Helsinki. MIBS and HIGS are registered (NCT00231751 and NCT00166387, respectively) at www.clinicaltrials.gov.
The Combined Cohort contains 104 brother pairs in which one brother had a current or history of an inhibitor and the other did not. Hereafter referred to as discordant pairs, these included 77 pairs from HIGS, 23 from MIBS, and 4 from HGDS. If a family contained more than 2 brothers with hemophilia, the oldest brother was chosen first and the next oldest brother with the opposite inhibitor status was then selected to complete the pair.
Multiple subjects were enrolled in more than 1 of the 3 studies. These were identified centrally and confirmed by site staff. In the case of duplicates, samples and clinical data collected as part of HIGS participation were used.
Clinical data collection
Demographic data including date of birth, race/ethnicity, and relationship to other participants were collected. Severe hemophilia was defined as factor VIII (FVIII) activity < 0.01 IU/mL, moderate as 0.01 to < 0.05 IU/mL, and mild as 0.05-0.40 IU/mL. Inhibitor-related variables, including presence or absence of a history of inhibitor and peak and current titer expressed as Bethesda units (BU) were measured and recorded as described previously.15 For this analysis, an inhibitor was defined as a current or history of a titer ≥ 1 BU measured at the local laboratory. This cutoff point allowed for consistent classification of inhibitor status across the 3 study groups included in the Combined Cohort.
F8 mutation typing
Standard methods for the analyses of the F8 gene mutation were used16 in HIGS and MIBS. In HGDS, the presence or absence of an inversion mutation (inversion/no inversion) was determined for 58% of the HGDS cohort.17 The remaining HGDS samples were mutation typed using the methods of Oldenburg.16 The following F8 gene mutations were categorized as high risk: inversions, large deletions, nonsense, small deletions/insertions (outside A-runs), missense (Arg593Cys, Tyr2105Cys, Arg2150His, Arg2163His, Trp2229Cys, Pro2300Leu, and Asn2286Lys), and splice site (at conserved nucleotides at position + or −1 and 2). Those considered low risk were: small deletions/insertions (within A-runs), splice site (at position + or −3 or more remote), missense (other regions), or other mutation types based on data from the Hemophilia A Mutation, Structure, Test and Resource Site (HAMSTeRS) database (http://hadb.org.uk), a resource site for study of FVIII genetic variation, and unpublished data from the Bonn Center in Germany.
Genotyping
An Illumina iSelect platform was used to genotype 14 626 SNPs (supplemental Appendix 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) from a set of 1081 genes. The genes (chiefly immune response and immune modifier genes) and cytokines, cytokine receptors, chemokines, chemokine receptors, immune and inflammatory pathway genes, and HLA genes were selected from a literature review of inflammatory and immune genes and pathway public databases. SNPs were selected from a region spanning 5 kb upstream and 1 kb downstream of the target genes using data for Yoruba (YRI) from Nigeria and CEPH Europeans from Utah (CEU) from the International HapMap Project (http://www.hapmap.org). We first selected all known or putative functional coding region (nonsynonymous, insertion, deletions, frameshift) and regulatory SNPs. Functional status was vetted using information from the National Center for Biotechnology Information (NCBI), PupaSuite (http://pupasuite.bioinfo.cipf.es), and SNPEffect (http://snpeffect.vib.be/index.php). SNPs that are reported to affect amino acid composition or to occur in splice sites, exonic splicing enhancer, exonic splicing silencer, or regulatory regions were selected as long as they met minimum Illumina platform restrictions. Using these as the first seeds, we added tagging SNPs (r2 > 0.8) equally spaced across the targeted gene region. Because of the paucity of exomic SNPs, most of the SNPs were located on introns or noncoding regions. Genotypes that exhibited significant deviation from Hardy-Weinberg equilibrium (P < .001) or that exhibited “missingness” greater than 20% were removed.
Statistical analysis
Population stratification was adjusted for using additional ancestry information generated by the Illumina panel to genetically determine race/ethnicity, allowing a more powerful method of classifying ancestry than data obtained by self-reported cultural identity or race. Using the genotypic data produces principal components that account for genetic variability; components were constructed using a subset of unrelated subjects and projected for the remaining subjects using EIGENSOFT.18 The first 3 principal components were included in the model as continuous covariates.
Combined cohort.
The analysis was performed separately for each cohort because of the varying distributions of related subjects and differences in inclusion criteria for each study. The HGDS cohort was analyzed with logistic regression, MIBS with Generalized Estimating Equations, and HIGS with alternating logistic regression. The outcome variable was defined as the absence or presence of a current or history of an inhibitor. Covariates included severity of hemophilia for MIBS and HGDS (mild, moderate, or severe), year of birth, genetically identified race, F8 gene mutation risk category in the case of MIBS and HIGS, and an indicator of presence of intron 1 or 22 inversion mutation for HGDS. Several of these covariates have been described previously as having an association with inhibitor development.1,2 Year of birth was included to adjust for potential differences in environmental or treatment practices that may have affected risk over the period of enrollment of the 3 cohorts: 1989-2010. Each variable was investigated for possible colinearity or correlation with the genotypic scores. Determining which covariates exhibited independence from genotypes ensured that the overlap in variance in the fitted model attributed to each predictor was limited. The primary hypothesis test was performed using the SNP as the main effect of interest and was modeled in an additive fashion with the number of minor alleles scored for each participant. Additional models were constructed for subsets of subjects with severe hemophilia A and for subjects with an inversion mutation.
Once estimates and standard errors were obtained for each cohort model and SNP, they were combined using a meta-analytic technique, summing the estimates weighted by the standard errors. A χ2 statistic was obtained and tested for the Combined Cohort.19 Selection of SNPs of interest was performed to identify markers that exhibited effects in the same direction in all 3 cohorts or allowing for a 20% interval around an odds ratio (OR) = 1 in 1 of the 3 to allow for random variation, as well as having a meta P < .001.
Discordant pairs.
A stratified logistical regression analysis was performed on the discordant pairs using a family identifier as the stratification variable to create the matched pairs. The year of birth was the only covariate in the analysis. The discordant pair models were performed similarly to the primary models, where the SNP was included as an additive effect, summing the number of the minor alleles.
Unless otherwise specified, the analyses were performed using SAS Version 9.2 software.20
Results
Of the 833 people enrolled in the Combined Cohort, 88% had severe, 8% moderate, and 4% mild hemophilia. Fifty-five percent (n = 457) had a current or history of an inhibitor. The demographic variables are shown by cohort in Table 1. Of 14 626 markers on the Illumina panel, 13 952 (95%) were successfully genotyped. A total of 13 331 passed the selection criteria and Hardy-Weinberg equilibrium testing.
Combined cohort
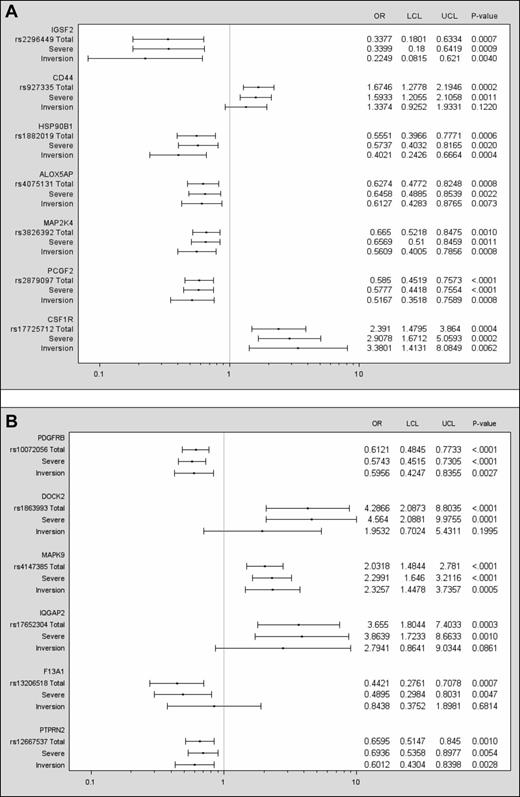
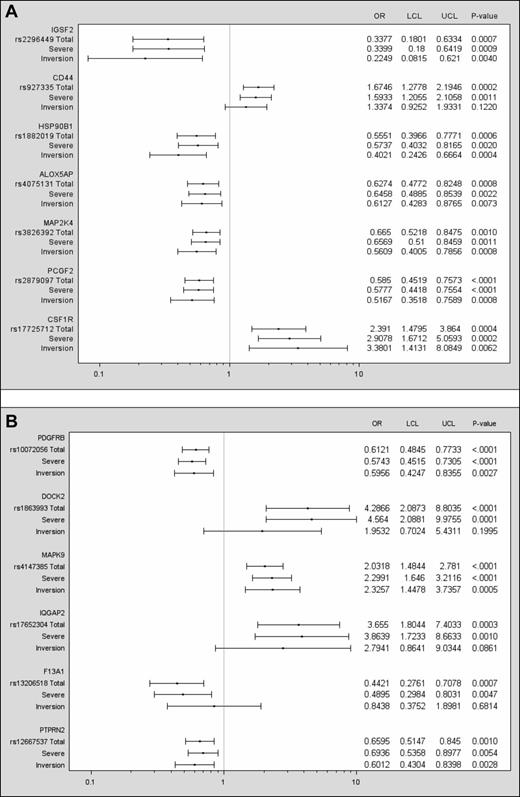
Fifty-three SNPs were found to be significant using the criteria of odds in the same direction, or allowing for a 20% interval around an OR = 1 in 1 of the 3 and significance in at least 2 cohorts (supplemental Appendix 2). Selection of those with a meta P < .001 reduced the list to 13 markers (Table 2 and Table 3); 8 of these showed protective effects compared with 5 exhibiting potential risk for inhibitor development. The 5 SNPs associated with an increased risk of inhibitor development are located on the genes CD44, CSF1R, DOCK2, MAPK9, and IQGAP2. Figure 1 is a graphic representation of the 13 markers showing results for 3 groups: (1) the total cohort, (2) those with severe hemophilia (approximately 90% of the total), and (3) those with the inversion mutation (approximately 50% of the total). Findings for the severes varied very little from those observed in the total cohort. In most cases, associations were significant for the subset with inversion mutations as well. For this group, however, the confidence intervals were generally wider, reflecting the decrease in sample size. With a more general correction method, the number of independent tests was determined as described by Gao, Starmer, and Martin.21 Using a 99% threshold from the principal components, the effective number of tests was determined to be 10 261, which results in a suggested threshold of 9.75 × 10−5 and a Sidak correction at 5.0 × 10−6.
SNPs having a meta P < .001, having odds in the same direction in all 3 cohorts, or allowing for a 20% interval around an OR = 1 in 1 of the 3 and 2 cohorts having significance at α = 0.05. Data are presented for the total cohort and for the severe and inversion mutation subsets. The left column lists the gene, the rs number of the SNP, and lines for results for the total cohort (N = 833), the subset with severe hemophilia (n = 733), and the subset with a F8 inversion mutation (n = 402). The ORs and confidence intervals are plotted for each group. A vertical line drawn from the x-axis indicates an OR = 1. The right columns show the numeric values for the ORs, lower and upper confidence limits, and P values.
SNPs having a meta P < .001, having odds in the same direction in all 3 cohorts, or allowing for a 20% interval around an OR = 1 in 1 of the 3 and 2 cohorts having significance at α = 0.05. Data are presented for the total cohort and for the severe and inversion mutation subsets. The left column lists the gene, the rs number of the SNP, and lines for results for the total cohort (N = 833), the subset with severe hemophilia (n = 733), and the subset with a F8 inversion mutation (n = 402). The ORs and confidence intervals are plotted for each group. A vertical line drawn from the x-axis indicates an OR = 1. The right columns show the numeric values for the ORs, lower and upper confidence limits, and P values.
Eight participants in the study group were reported to have historical maximum BU titers between 0.6 and < 1. A sensitivity analysis supported the results for the 13 SNPs: after removing these subjects from the study group, the direction of the OR for the SNPs was the same and the maximum difference in the OR magnitude was 0.07 (SNP rs1882019, 0.52 in the total cohort compared with 0.55 in the sensitivity analysis). The P values for these SNPs were also very similar, with a maximum difference of .0012.
An evaluation of those with high responding (> 5 BU, 79.6%) inhibitors yielded similar results to those observed in the total group (data not shown). The group with low-response (≤ 5 BU, 20.4%) inhibitors was too small to examine separately. Given the relative racial/ethnic homogeneity in this population, it was not possible to examine the effects of the SNPs among groups with differing racial ancestry.
The effect of the F8 mutation on inhibitor risk was evaluated in the models for the HIGS and MIBS cohorts. This was not possible in the HGDS because the size of the group in this cohort that was typed beyond the presence or absence of the inversion mutation (n = 71) did not provide sufficient power to reflect the estimate of the effect of F8 mutation. For the HIGS cohort, the effect of having a high- versus a low-risk F8 mutation was a median OR = 2.05 (interquartile range, 2.03-2.08) independent of the other covariates and the SNP of interest. For the MIBS cohort, the median OR = 1.88 (interquartile range, 1.79-1.91).
There were 6 nonsynonymous markers located on the F8 gene included in the analysis. These were not genotyped with the iSelect panel, but were part of a subset of additional markers typed in the study group. The effect of each marker on the risk of inhibitor was tested. None showed significant associations in our population.
Discordant pairs
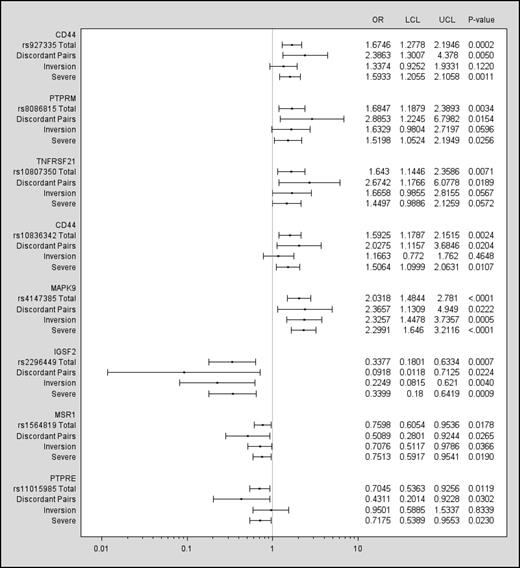
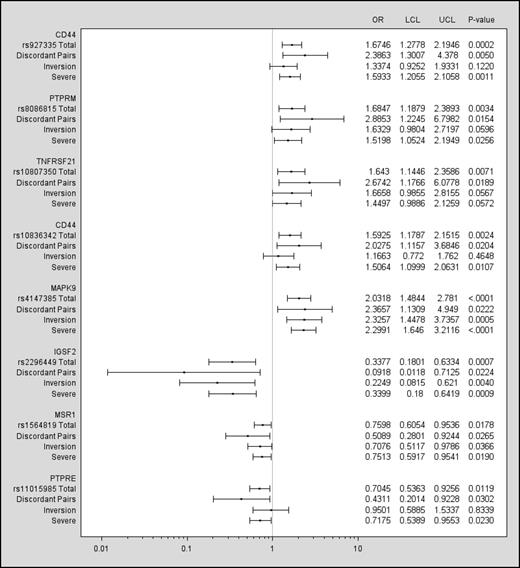
Eight of the 53 SNPs (Figure 2 and Table 3) were also significant predictors of inhibitor status at a P < .05 level among the discordant pairs. These SNPs included locations on CD44 (2 SNPs), PTPRM, TNFRSF21, MAPK9, IGSF2, MSR1, and PTPRE. The ORs for all 8 SNPs were in the same direction as in the total cohort, further supporting the results of the overall analysis.
SNPs significant in discordant pairs (P < .05). Data are presented for the discordant pairs, the total Combined Cohort, and severe and inversion mutation subsets of the Combined Cohort. The left column lists the gene, the rs number of the SNP, and lines for results for the discordant pairs (n = 104), total cohort (N = 833), the subset with severe hemophilia (n = 733), and the subset with a F8 inversion mutation (n = 402). The ORs and confidence intervals are plotted for each group. A vertical line drawn from the x-axis indicates an OR = 1. The right columns show the numeric values for the ORs, lower and upper confidence limits, and P values.
SNPs significant in discordant pairs (P < .05). Data are presented for the discordant pairs, the total Combined Cohort, and severe and inversion mutation subsets of the Combined Cohort. The left column lists the gene, the rs number of the SNP, and lines for results for the discordant pairs (n = 104), total cohort (N = 833), the subset with severe hemophilia (n = 733), and the subset with a F8 inversion mutation (n = 402). The ORs and confidence intervals are plotted for each group. A vertical line drawn from the x-axis indicates an OR = 1. The right columns show the numeric values for the ORs, lower and upper confidence limits, and P values.
Discussion
Based on the findings to date, it is clear that the pathophysiologic immune process is complex and potentially impacted by both genetic and nongenetic factors. Because a primary focus of hemophilia care is identification of subjects at risk before the start of treatment, a more complete characterization of genetic markers associated with inhibitor development is warranted. The present study is an extensive effort to address this task, and several new candidates that are potentially predictive in the immune response to the deficient factor have been identified. It is the standard that genetic findings be replicated in different cohorts for secure validation, therefore, this study considered demographic, clinical, and laboratory data from 3 cohorts, each with a different study design and including the population-based HGDS, enrolled over a 20-year period (1989-2010). The cohorts were evaluated separately adjusting for family relationships, year of birth, genetically determined racial ancestry, hemophilia severity, and type of F8 mutation. The cutoff used for defining inhibitor status (≥ 1 BU) is somewhat above that typically used. Whereas this could have the effect of misclassifying those with very low titer inhibitors as “noninhibitors,” a sensitivity analysis showed that there was little difference in the results when subjects (n = 8) with maximum BU titers between 0.6 and < 1 were excluded from the analysis. Altogether, we identified 53 markers having significance in at least 2 of the 3 cohorts at P < .05 independent of the type of causative mutation and the other covariates. In 13 of the markers, the meta P < .001. In addition, to maximize the opportunity to challenge our findings by testing them in 3 cohorts of differing study design, we also tested the SNPs in a subset of brother pairs discordant for inhibitor status. The functions of the most significant markers, including the 8 markers significantly associated with inhibitor risk in the discordant pairs, are briefly summarized. Given minimization of other intervening factors, the subgroup of inhibitor discordant brother pairs are of particular interest. Although not considering potential nongenetic factors, the observation of consistent significant associations between the markers on these genes strongly suggest that they are important modifiers of the immune response. The majority of markers are known to be involved in various B and/or T cell–mediated mechanisms and several of the genes code for molecules involved in intracellular signaling pathways, many of which may interact with one another. Our study was designed to test the most likely candidate genes given their physiologic function. Other common variants are located near nonimmune-related genetic regions; however, the initial selection focused on the most likely related genes. Different follow-ups may be proposed that capture pathways traditionally not associated with immune-related responses. It is also worth noting that the number of unique combinations of marker subsets is nearly infinite in size. In terms of familial rare inherited SNPs, cohorts of brother pairs have limited power to detect this type of association. However, our planned follow-up study of family trios (mother, father, and affected offspring) is designed for this.
Interestingly, in a sizable number of cases (n = 27; 50.9%), the identified effects of the minor alleles were protective, suggesting that the SNPs exert an effect on the gene increasing the capability to down-regulate the immune response to the deficient factor. Therefore, it is apparent that down-regulatory markers may be important and even override the effect of SNPs up-regulating the activity of immune regulators and promoting the response. These mechanisms may also modify the risk for inhibitor development associated with immune system challenges, explaining why events such as major surgery and severe trauma may promote inhibitor formation in some, but not all, patients independently of the severity of the disease and why prophylaxis given at young age may influence inhibitor risk.22 Again, the present analysis has not considered treatment-related factors, which may in some cases modulate the inhibitor risk.
The SNPs located in immunoregulatory genes previously associated with inhibitor risk in independent cohorts6-12 were not consistently associated with risk in this investigation and in the meta-analysis combining all 3 cohorts. Among these previously described candidates, the SNP at location −1082A/G in the promoter region of IL-10 is the most frequently reported finding. In our study, the ORs for this SNP ranged from 0.8-1.3 with a meta P = .22. The absence of supporting effects in our cohorts may be due to composition differences between HIGS and previously reported populations. In the case of the IL-10 SNP, the microsatellite in the IL-10 gene associated with inhibitor risk in the MIBS study was not included in this SNP panel.11 Other possible explanations are the variation and low frequencies of inhibitor-associated minor alleles and genotypes, such as the A allele at −308 in TNFA identified in 2.3%-17.3% of subjects within the HapMap project depending on ethnicity, compared with 13.6% in our Combined Cohort. The discrepancy between previously reported populations and our cohort and the low frequencies are also reflected in the homozygous genotypes of the associated SNPs: < 2% for the A-allele at −308 in TNFA. In the case of the T allele at −318 in CTLA4, the reported frequencies are even less. The low magnitude and change in frequency potentially affect the power to detect significant associations. Finally, interaction with nongenetic factors, another potential reason, was not tested in this analysis.
Fully appreciating the importance of these findings for the development of inhibitors will require considerably more research. However, our present results support the complexity of the immune response and encourage further research with the goal of understanding the pathways involved—more so than single genetic markers. Whether some pathways will be more important than others is not clear at this stage, but our results suggest that several potential therapeutic targets may be identified in the future for a variety of Ab-mediated diseases, including inhibitor development in hemophilia. The data also support the theory that the formation of Abs will be influenced by the combination of a genetic predisposition and immune system challenge(s) in a substantial number of patients with the appropriate subset of immunocompetent cells and HLA class II alleles. Furthermore, our findings may be used to define a risk score based on a combination of additive genetic markers. Work toward that goal is currently ongoing through an evaluation of the impact of the most significant findings from the candidate gene panel and including those previously reported. This score will then be validated in yet another independent cohort to determine its value in the clinical setting and the possibility of individualized treatment to minimize inhibitor development.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the participants and parents who volunteered to participate in these studies; George W. Nelson (Frederick National Laboratory for Cancer Research) for contributions to the design of the HIGS; Jen Waters, Alice Lail, and Kaitlyn McConville (Rho Inc) for data collection, management, and statistical support; and Elizabeth Binns-Roemer (Frederick National Laboratory for Cancer Research) for management of study samples and genotyping.
The authors further acknowledge the participating HIGS investigators and centers in order of contribution: Raina Liesner, Great Ormond Street Hospital for Children National Health Service Trust, London, United Kingdom; Jerzy Windyga and Anna Klukowska, Institute of Hematology and Blood Transfusion, Warsaw, Poland; Kaan Kavakli, Ege University Hospital, Izmir, Turkey; Elena Santagostino and Maria Elisa Mancuso, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy; Donna DiMichele and Patricia Giardina, Weill Cornell Medical College, New York, NY; Georges Rivard, Hôpital Ste-Justine, Montreal, QC; Johannes Oldenburg, University Clinic Bonn, Bonn, Germany; Marijke van den Berg and R. Schutgens, University Medical Center Utrecht, Utrecht, Netherlands; Nadia Ewing, City of Hope National Medical Center, Duarte, CA; Jan Astermark, Center for Thrombosis and Hemostasis, Lund University, Skåne University Hospital Malmö, Malmö, Sweden; Anne Mäkipernaa, Clinical Research Institute Helsinki, Helsinki, Finland; Rosemary Schwyzer, Johannesburg Hospital, Johannesburg, South Africa; Amy Shapiro, Indiana Hemophilia and Thrombosis Center, Indianapolis, IN; Carmen Altisent, Hospital Vall d'Hebron, Barcelona, Spain; Raúl Peréz Bianco, Academia Nacional de Medicina, Buenos Aires, Argentina; Jonathan Ducore, University of California, Davis, Sacramento, CA; Cindy Leissinger, Louisiana Comprehensive Hemophilia Care Center, Tulane University, New Orleans, LA; Arlette Ruiz-Sáez, Centro Nacional de Hemofilia, Caracas, Venezuela; Peter Collins, Arthur Bloom Hemophilia Center, Cardiff, Wales; Paul Monahan, University of North Carolina Comprehensive Hemophilia Center, Chapel Hill, NC; Marjolein Peters, Academisch Medisch Centrum, Amsterdam, The Netherlands; Leonard Valentino, Rush University Medical Center, Chicago, IL; Mayte Alvárez and Victor Jíminez-Yuste, La Paz University Hospital, Madrid, Spain; Elizabeth Chalmers, Royal Hospital for Sick Children, Glasgow, Scotland; Romualdas Jurgutis, Klaipedos Jurininku Ligonine, Klaipeda, Lithuania; Peter Kouides, Rochester General Hospital, Rochester, NY; Hartmut Pollman, Hemophilia Center and Institute for Thrombosis and Hemostasis, Münster, Germany; Courtney Thornburg, Duke University, Durham, NC; James Huang, University of California, San Francisco, San Francisco, CA; Christoph Male, Medizinische Universität Wien, Vienna, Austria; Páll Önundarson, Landspitali University Hospital, Reykjavik, Iceland; María Helena Solano, Hospital San Jose, Bogota, Colombia; M. H. Cnossen, Erasmus Medical Center, Rotterdam, The Netherlands; Miguel Escobar, University of Texas Health Science Center at Houston, Houston, TX; Edward Gomperts, Children's Hospital Los Angeles, Los Angeles, CA; Rathi Iyer, University of Mississippi Medical Center, Jackson, MS; Michael Makris, Sheffield Hemophilia and Thrombosis Center, Royal Hallamshire Hospital, Sheffield, United Kingdom; Savita Rangarajan, Guy's and St Thomas's National Health Service Foundation Trust, London, United Kingdom; Indira Warrier and Meera Chitlur, Children's Hospital of Michigan, Detroit, MI; Philippe de Moerloose, Hôpital Universitaire de Genève, Geneva, Switzerland; Gillian Evans, Kent and Canterbury Hospital, Canterbury, United Kingdom; Ralph Gruppo, Cincinnati Children's Hospital Medical Center, Cincinnati, OH; Dragana Janic, University Children's Hospital, Belgrade, Serbia; and Dragan Micic, Mother and Child Health Care Institute of Serbia Dr Vukan Cupic, Belgrade, Serbia.
This work was supported by an investigator-initiated grant from Baxter BioScience and by federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health (NIH; contract number HHSN261200800001E). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The Malmö International Brother Study is funded through grants from Wyeth and the Research Fund at Malmö University Hospital. The Hemophilia Growth and Development Study is funded by the NIH, National Institute of Child Health and Human Development (grant R01-HD-41224).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: J.A. and E.B. designed and performed the research, collected, analyzed, and interpreted the data, and wrote the manuscript; S.M.D. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript; E.D.G., J.O., B.K., D.M.D., and A.D.S. designed and performed the research and collected the data; J.S. and E.D.M. designed and performed the research, analyzed and interpreted the data, performed the statistical analysis, and wrote the manuscript; A.P. performed the research and analyzed and interpreted the data; and C.A.W. designed and performed the research, collected and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.M.D. is Division of Blood Diseases and Resources, National Heart, Lung, and Blood Institute, NIH, Bethesda, MD.
Correspondence: Jan Astermark, MD, PhD, Centre for Thrombosis and Haemostasis, Lund University, Skåne University Hospital, SE-205 02 Malmö, Sweden; e-mail: jan.astermark@med.lu.se.