To the editor:
Recently, the frequently mutated gene MLL3 was found to be related to the pathogenesis of hepatocellular carcinoma (HCC), fluke-associated cholangiocarcinoma, gastric cancer, and transitional carcinoma of the bladder,1-4 raising the possibility that the MLL3 gene and its encoded chromatin remodeling protein MLL3 are etiologically related to cancers. We performed exome sequencing for 4 patients in a multigenerational pedigree with colorectal cancers and acute myeloid leukemia (AML) and identified an insertion mutation in the MLL3 gene on chromosome 7, producing a frame shift leading to a premature truncation at codon 827. To our knowledge, it was the first germ line MLL3 mutation found in a cancer pedigree. Because MLL3 is an enzyme for histone methylation, pharmacologic intervention may be possible.
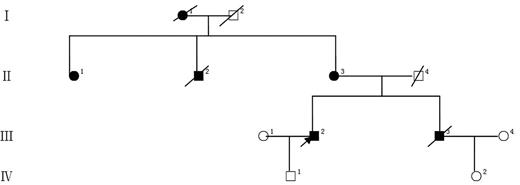
Of the 4 patients we analyzed in this multigenerational pedigree (Figure 1), II-1 was diagnosed with rectal cancer at age 43 and still alive at age 56; II-3 was diagnosed with colon cancer at age 59 and still alive at age 67; III-2 was diagnosed with AML-M2 at age 40 and still alive at age 45 in complete remission state; and III-3 was diagnosed with AML-M1 at age 43 and died at age 43. All subjects gave informed consent, and the protocol was approved by the Committee on Studies Involving Human Beings at Tianjin Medical University and Southwest Hospital of Third Military Medical University. Genomic DNA was extracted from whole blood samples; exome sequencing was carried out for these 4 patients, respectively. A heterozygous insertion mutation in the MLL3 gene on chromosome 7 (151,945,071 bp, ins T; Human Genome assembly GRCh37/hg19, genome.ucsc.edu) was identified in both the colorectal patients and the AML patients. The insertion, which started at codon 817 in exon 14, results in a frame shift mutation of MLL3, leading to a premature stop codon “TAA” at codon 827.
A Chinese pedigree with colorectal cancers and acute myeloid leukemia. Solid symbols indicate affected individuals. Open symbols indicate unaffected individuals. The arrow indicates the proband, and slashes indicate deceased persons. I-1, lung cancer. II-1, rectal cancer. II-2, rectal cancer. II-3, colon cancer. III-2 and III-3, acute myeloid leukemia, M2 and M1.
A Chinese pedigree with colorectal cancers and acute myeloid leukemia. Solid symbols indicate affected individuals. Open symbols indicate unaffected individuals. The arrow indicates the proband, and slashes indicate deceased persons. I-1, lung cancer. II-1, rectal cancer. II-2, rectal cancer. II-3, colon cancer. III-2 and III-3, acute myeloid leukemia, M2 and M1.
MLL3, which belongs to the human TRX/MLL family, is an important mammalian H3K4 methyltransferase. Down-regulation of MLL3 promoted cell proliferation in HCC cell lines.1 Homozygous MLL3− knockout mice display tumors in the innermost layer of ureter cells.5 These results suggest that MLL3 is a tumor suppressor.
Mechanistically, the MLL3 protein can selectively recognize H3K4me and affect the mechanistic readout of histone tail modifications.6 In addition, ASCOM-MLL3 has a redundant but crucial role in transactivation of p53 and participates in DNA-damage-induced expression of p53-targeted genes.5 In addition, nuclear-receptor-mediated downstream gene expression was regulated by MLL3.7
Because MLL3 is an enzyme, pharmacologic intervention may be possible. Drugs targeting this molecule may be effective in both colorectal and AML with MLL3 mutation. The restoration of balance between histone methylation and demethylation may facilitate current tumor therapy.
Authorship
Acknowledgments: The authors thank Xiao-Bo Tian, Da-Bing Qin, and Yu Liu for case management and history taking. This work was supported by the National Natural Science Foundation of China (NSFC 81270605, 30971066, 81070576), Third Military Medical University Clinical and Science Great Fund Project (2101XLC03), Chongqing Postgraduate Education Reform Project (yjg123114), and Chongqing Natural Science Fund Project (CSTC'2008BA5001)
Contribution: J.-P.C. and W.-D.L. conceived the study; Q.-R.L. and F.-J.W. performed the experiments; and J.-P.C., W.-D.L., and S.-N.X. wrote the manuscript with input from Z.-J.Y. and J.-K.C.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jie-Ping Chen, Department of Hematology, Southwest Hospital, Third Military Medical University, Chongqing 400038, China; e-mail: chenjpxn@yahoo.com.cn.
References
Author notes
W.-D.L., Q.-R.L., and S.-N.X. contributed equally to this work.