In this issue of Blood, Riches and colleagues provide an in-depth characterization of T cells and particularly CD8+ T cells from patients with chronic lymphocytic leukemia (CLL). They demonstrate that CD8+ T cells exhibit defects in proliferation, cytotoxicity, and increased expression of inhibitory receptors and thus exhibit features of T-cell exhaustion.1
Naive CD8+ T cells differentiate to effector CD8+ T cells after viral infections. Antigen exposure leads to the differentiation to memory CD8+ T cells, which can persist long term and proliferate rapidly with reinfection.
In the course of chronic infections, CD8+ T cells become “exhausted” with poor effector function and the expression of multiple inhibitory receptors (eg, PD-1, CTLA4). Exhausted T cells have low proliferative capacity and cannot persist without antigens.2 CD8+ T-cell exhaustion appears to be a prominent feature of many experimental models of chronic infections, but has also been demonstrated in humans with chronic infections as well as cancer.2 Despite the importance of CD8+ T-cell exhaustion, the underlying molecular mechanisms remain incompletely understood (reviewed by Wherry2 ).
Recent studies suggest that T-cell exhaustion is an active, tightly regulated process with controlled and stepwise regulation of inhibitory cell surface receptors (eg, PD-1, Lag-3, Tim-3) and cytokines, such as IL-10 and TGF-β.2 The existence of active and regulatory pathways points to the possibility of restoring T-cell function of “exhausted” cells, which would have immediate clinical potential.3 Indeed, early clinical trials blocking the PD-1 pathway show promise in solid tumors and are being explored in hematologic malignancies.4,5
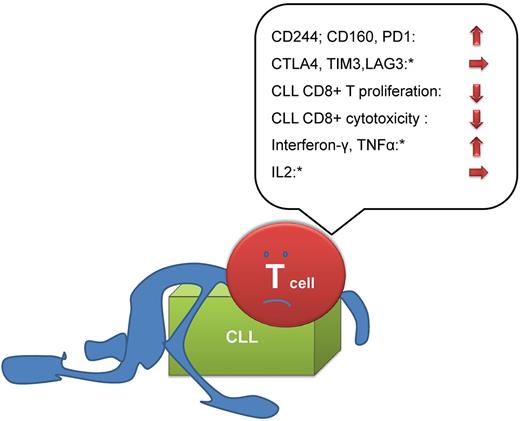
In a set of thorough and well-structured experiments, Riches and colleagues compared the properties of T cells in normal volunteers (age matched) and CLL patients. The authors add to the existing literature on T-cell defects in CLL by showing increased expression of the exhaustion markers CD244, CD160, and PD1 in T cells from CLL patients. Other markers of exhaustion were not increased (CTLA4, TIM3, and LAG3) in CLL. The further analysis of T-cell subsets suggested that the circulating T-cell compartment in CLL is shifted toward an increased proportion of antigen-experienced T cells with some phenotypic features of exhausted T cells. When the authors analyzed the functional properties of these cells they found decreased proliferation and ability to kill target cells ex vivo.
In contrast to the hierarchical loss of cytokine production in viral infection–associated exhaustion,2 CD3+CD8+ T cells from CLL patients had increased production of IFNγ and TNFα, and reduction in IL2 production.
Because CMV serum positivity has a profound influence on the major lymphoid subsets, it was important to show that the skewed T-cell function was independent of CMV infection. Following up on prior work of the group showing impaired immunologic synapse formation in CLL,6 the authors go on to demonstrate that CLL patients show defective cytotoxicity because of failure of granzyme localization to the immunologic synapse.
While some of the traits described by the authors are in keeping with “classical exhaustion” as demonstrated in chronic viral infection, the overall analysis reveals that some traits (cytokine profile, expression of other exhaustion markers) are different in CLL (see figure). The authors speculate that a “pseudo-exhausted” state of CLL T cells may be due to the recently identified chronic stimulation by autonomously active signaling of the B-cell receptor characteristic for CLL.7
“Exhaustion” or “pseudoexhaustion” in CLL. Key findings of Riches et al are summarized. While a number of traits (high expression of CD244, CD160, PD-1; impaired proliferation/cytotoxicity) argue for the existence of exhaustion in CLL, not all characteristics of exhausted T cells (eg, cytokine production) are shared in CLL (*).
“Exhaustion” or “pseudoexhaustion” in CLL. Key findings of Riches et al are summarized. While a number of traits (high expression of CD244, CD160, PD-1; impaired proliferation/cytotoxicity) argue for the existence of exhaustion in CLL, not all characteristics of exhausted T cells (eg, cytokine production) are shared in CLL (*).
As increasing data on T-cell exhaustion in viral infections becomes available (2 different types of viral-specific CD8+ cells, which were defined by their expression levels of the T-box transcription factors T-bet and eomesodermin [Eomes] were recently shown to be important for viral control8 ), much remains to be learned from comparing cancer and infection scenarios as well as immune response in different cancer types.
Based on the work of Riches et al it will be important to understand the contribution of the observations for the diverse alterations of the immune system in CLL as autoimmune phenomena or response to treatment (eg, Imids).9,10 This work also opens up potential routes to exploit “exhaustion” or “pseudoexhaustion” therapeutically by pharmacologic targeting of inhibitory receptors such as PD-1.4,5
Conflict-of-interest disclosure: The author declares no competing financial interests. ■