Key Points
Human FcγRI can trigger antibody-induced inflammatory arthritis, thrombocytopenia, airway inflammation, and systemic anaphylaxis.
Human FcγRI can trigger antibody-mediated immunotherapy of mouse metastatic melanoma.
Abstract
Receptors for the Fc portion of IgG (FcγRs) are mandatory for the induction of various IgG-dependent models of autoimmunity, inflammation, anaphylaxis, and cancer immunotherapy. A few FcγRs have the ability to bind monomeric IgG: high-affinity mouse mFcγRI, mFcγRIV, and human hFcγRI. All others bind IgG only when aggregated in complexes or bound to cells or surfaces: low-affinity mouse mFcγRIIB and mFcγRIII and human hFcγRIIA/B/C and hFcγRIIIA/B. Although it has been proposed that high-affinity FcγRs are occupied by circulating IgG, multiple roles for mFcγRI and mFcγRIV have been reported in vivo. However, the potential roles of hFcγRI that is expressed on monocytes, macrophages, and neutrophils have not been reported. In the present study, we therefore investigated the role of hFcγRI in antibody-mediated models of disease and therapy by generating hFcγRI-transgenic mice deficient for multiple endogenous FcRs. hFcγRI was sufficient to trigger autoimmune arthritis and thrombocytopenia, immune complex-induced airway inflammation, and active and passive systemic anaphylaxis. We found monocyte/macrophages to be responsible for thrombocytopenia, neutrophils to be responsible for systemic anaphylaxis, and both cell types to be responsible for arthritis induction. Finally, hFcγRI was capable of mediating antibody-induced immunotherapy of metastatic melanoma. Our results unravel novel capabilities of human FcγRI that confirm the role of high-affinity IgG receptors in vivo.
Introduction
Receptors for the Fc portion of IgG (FcγRs) are expressed in humans and mice and mediate most biologic activities of IgG antibodies. FcγRI (CD64), FcγRIIB (CD32B), and FcγRIIIA (CD16A) exist in both species. FcγRIIA (CD32A), FcγRIIC (CD32C), and FcγRIIIB (CD16B) are specific to humans, whereas FcγRIV is specific to mice. This nomenclature is based on amino acid sequence homology, but does not systematically reflect functional homologies or similar expression patterns between FcγRs in both species.1 Therefore, the role of human FcγRs may not be predicted from the role of their homologs studied in mice. Transgenic mice expressing human FcγRs (hFcγRs) have been generated to enable their analysis in disease and therapy models in vivo. Whereas hFcγRIIA has been extensively studied using transgenic mice,2-5 some hFcγRs, such as hFcγRI, have been intriguingly understudied in vivo.
hFcγRI is the only high-affinity IgG receptor in humans. It binds human IgG1, IgG3, and IgG4 with a high affinity and has no affinity for IgG2.6 High-affinity FcγRs (KA ∼ 107-108M−1 for IgG), but not low-affinity FcγRs (KA ∼ 105-106M−1 for IgG), are defined by their ability to bind IgG as monomers. Both types of FcRs bind IgG when present in immune complexes (ICs) or when opsonizing cells or surfaces. Therefore, high-affinity FcγRs are thought to be occupied/saturated by IgG in vivo, leading to the belief that prebound IgG prevents the participation of high-affinity receptors in IC-mediated reactions. Inversely, low-affinity FcγRs are believed to remain free and thus to be responsible for IC-mediated reactions. ICs, however, have been reported to displace monomeric IgG from high-affinity FcγRs within minutes.7 Furthermore, even when in the presence of elevated IgG levels in vitro, high-affinity FcγRs have been reported to retain their ability to bind opsonized RBCs.8 It could thus be demonstrated that the mouse high-affinity IgG receptor mFcγRI contributes to inflammation severity in multiple models of disease.9-14 The expression pattern of mFcγRI has been a recent matter of debate. Expression on monocytes and on thioglycolate-elicited macrophages have been reported by some10,11,15 and contradicted by others,16,17 whereas all report expression on BM-derived macrophages and no expression on neutrophils. Low levels of FcγRI have been reported on dendritic cells (DCs) from normal thymi, with higher levels in the spleen, lymph nodes, and skin-emigrant DCs,16 and on CD11b+CD11c+MHCII+Ly6C+ monocyte-derived skin DCs.18 However, the cells responsible for the contribution of mFcγRI to disease models remain to be identified. No role for mFcγRI could, however, be identified in the passive model of antibody-induced inflammatory arthritis (K/BxN),19 antibody-induced immune thrombocytopenia,20 or active systemic anaphylaxis (ASA).21 It could nevertheless be demonstrated that the other mouse high-affinity IgG receptor, mFcγRIV, which is expressed on monocyte/macrophages and neutrophils, contributes to several of these models of autoimmunity, inflammation, and anaphylaxis.17,21,22
The human homolog of mFcγRI, hFcγRI, is expressed on blood monocytes and tissue macrophages.23,24 Under many circumstances, including chemotherapy, multiple myeloma,25 rheumatoid arthritis,23 bacterial infection, sepsis, inflammatory bowel disease, or treatment with recombinant G-CSF, hFcγRI is also expressed by neutrophils. Therefore, the expression pattern of hFcγRI and mFcγRI appear to be different, suggesting that their roles in pathology and therapy may also be very different. Whereas a role for hFcγRI on DCs has been reported in enhancement of antigen presentation and cross-presentation,24 its role(s) on monocytes, macrophages, and neutrophils has not been addressed. Monocytes/macrophages have been involved in IC-induced airway inflammation,26 in antibody-dependent cellular cytotoxicity of opsonized platelets leading to thrombocytopenia,15 and of opsonized tumor cells in mouse models of metastatic cancer.12 Monocytes/macrophages also induce neutrophil recruitment into inflamed tissue such as joints or pulmonary tissues in models of inflammatory arthritis27 or airway inflammation,28 respectively. Neutrophils have been reported to be mandatory for the induction of inflammatory arthritis29 and IC-dependent airway inflammation and, recently, to contribute to models of systemic anaphylaxis.2,21
Mice transgenic for the Fcgr1a gene that recapitulate the expression of the high-affinity receptor hFcγRI in humans have been generated.1,30 Based on its expression pattern, we hypothesized that hFcγRI may be capable of inducing antibody-dependent autoimmunity, anaphylaxis, and tumor immunotherapy models to which monocytes/macrophages and/or neutrophils have been reported to contribute. We crossed hFcγRItg mice with mice deficient for multiple endogenous FcRs and found that hFcγRI bound several mouse IgG subclasses as monomers, thereby conserving its properties as a high-affinity receptor in vivo in these mice. In this context, we demonstrate that hFcγRI was sufficient to induce not only autoimmune arthritis, thrombocytopenia, airway inflammation, and fatal systemic anaphylaxis, but could also mediate the therapeutic efficacy of clinically adapted humanized antitumor antibodies on metastatic melanoma. Therefore, the human high-affinity IgG receptor hFcγRI might be a pro-inflammatory and pro-anaphylactic IgG receptor in humans that can mediate IgG-based antitumor immunotherapies.
Methods
Flow cytometric analysis
Blood cell populations were defined as follows: mouse B cells (CD19+), T cells (CD3+), monocytes/macrophages (blood/peritoneum: CD11b+/Gr1−; BAL: CD11c+/Gr1−), neutrophils (Gr1+/SiglecF−), basophils (IgE+/DX5+), eosinophils (Gr1int/SiglecF+), mast cells (IgE+/CD117+), platelets (DX5+/CD61+), and natural killer (NK) cells (NK1.1+/DX5+); human B cells (CD19+), T cells (CD3+), NK cells (CD56+), monocytes (CD14+) neutrophils (CD24+), basophils (CD123+/CD203c+), and eosinophils (CD24+/CD193+). Expression of different Flag-tagged FcRs in CHO-K1 cells was compared using anti-FLAG antibody.
IC binding.
CHO-K1 cells were incubated with preformed ICs made of 10 μg/mL of TNP5-BSA-biotin and 15 μg/mL of anti-TNP mAbs, for 1 hour at 4°C. Bound ICs were detected using PE-conjugated neutravidin at 2 μg/mL for 30 minutes at 4°C.
Monomeric Ig-binding assays.
CHO-K1 cells were incubated with 10μg/mL monomeric mIgG or rabbit IgG for 1 h at 4°C. Cell-bound Ig was detected using 5μg/mL PE-labeled F(ab′)2 fragments of antimouse F(ab′)2-specific or 15 μg/mL FITC-conjugated F(ab′)2 anti–rabbit Ig, respectively, for 30 minutes at 4°C.
Airway inflammation
Mice were injected intranasally with 20 μL of rabbit anti-ovalbumin (anti-OVA) antiserum and intravenously with 500 μg of OVA. After 18 hours, mice were lethally anesthetized and 4 bronchoalveolar lavages (BALs) of, respectively 0.5, 1, 1, and 1 mL of PBS were performed. The supernatant of the first BAL was used to quantify myeloperoxidase content. The cells from all BALs were pooled for cell-count analysis. Hemorrhage was determined in the cell-free supernatant of pooled BALs after RBC lysis by optical density measurement (570 nm).
Anaphylaxis
PSA.
For the passive systemic anaphylaxis (PSA) assay, ICs made of 80 μg of glucose-6-phosphate isomerase (GPI) and 200 μL of anti-GPI–containing serum (K/BxN serum) in 300 μL of physiologic solution were performed at 37°C and injected intravenously. Alternatively, 10-200 μg of antagonistic blocking anti-hFcγRI.1 or agonistic nonblocking anti-hFcγRI.2 mAb was injected intravenously. Central body temperature was recorded using a digital thermometer (YSI).
ASA.
For the ASA assay, mice were injected intraperitoneally on day 0 with 200 μg of BSA in complete Freund adjuvant and boosted intraperitoneally on days 14 and 28 with 200 μg of BSA in incomplete Freund adjuvant. BSA-specific IgG1, IgG2a/b/c and IgE antibodies in serum were titered by ELISA on day 30 as described previously.21 Mice with comparable antibody titers were challenged intravenously with 500 μg of BSA 8 days after the last immunization. Central temperature was monitored.
Lung metastases model
B16-Luc2+ cells (1 × 106) were injected intravenously on day 0, and anti–TYRP-1 mAbs TA99 (200 μg), CTA99 (500 μg), or human IgG1 anti–TYRP-1 (500 μg) were injected intraperitoneally on days 0, 1, 2, 4, 7, and 9. Shaved and anesthetized mice were injected intraperitonelly with 3 mg of luciferin 5 minutes before or explanted lungs were exposed to 50 μL of 15 mg/mL luciferin 2 minutes before bioluminescence acquisition on an IVIS 100 (Caliper Life Sciences), using 5-minute exposure times with medium binning. Total photon flux (photons/seconds) of the entire lung was calculated using Living Image Version 3.2 software.
Please refer to supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for information on mice, reagents, in vivo blocking and depletion, K/BxN serum-induced passive arthritis (K/BxN PA), experimental thrombocytopenia (ITP), surface plasmon resonance analysis, and statistical analyses. All mouse protocols were approved by the Animal Care and Use Committees of Paris, Ile de France, France.
Results
hFcγRI can trigger passive inflammatory arthritis
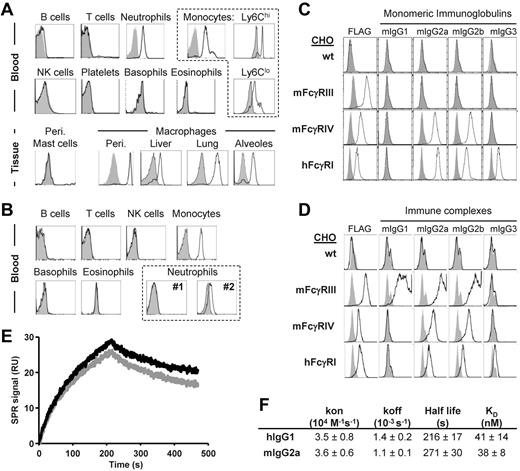
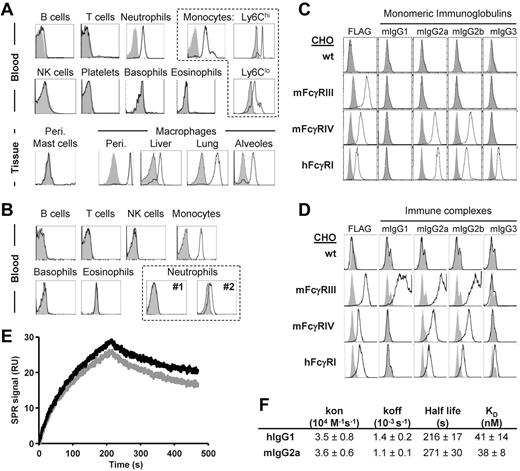
To investigate the pro-inflammatory potential of hFcγRI in vivo, we crossed mice transgenic for hFcγRI (hFcγRItg)30 to mice deficient for 5 endogenous FcRs (FcγRI/IIB/III−/− FcϵRI/II−/− mice, also referred to as 5KO mice).7 These mice still express the FcRγ-chain that is mandatory for hFcγRI expression and endogenous FcγRIV. In hFcγRItg 5KO mice, hFcγRI was expressed in the blood specifically on Ly6Chi and Ly6Clo monocytes; neutrophils; and peritoneal, liver, lung, and alveolar macrophages, but not on peritoneal mast cells (Figure 1A), which is consistent with a previous study.30 The expression pattern of hFcγRI in hFcγRItg 5KO mice therefore mimics its expression pattern in humans, where hFcγRI is constitutively expressed on monocytes and inducible on neutrophils. Whereas the expression level of hFcγRI was higher on neutrophils from these mice compared with human neutrophils from 2 different healthy donors, it was similar on mouse monocytes compared with monocytes from healthy donors (Figure 1B and supplemental Figure 1A). hFcγRI bound mouse IgG2a, IgG2b, and IgG3, but not mouse IgG1, either as monomers (Figure 1C) or as ICs (Figure 1D). Moreover, the analysis of the interaction of hFcγRI with mouse IgG2a or with human IgG1 resulted in similar association (kon) and dissociation (koff) rate constants, and therefore in a very similar calculated affinity constant (KD ∼ 40nM, ie, KA ∼ 2.5 × 107M−1; Figure 1E-F and supplemental Figure 1B). Therefore, hFcγRI retained its properties as a high-affinity receptor for IgG when expressed in transgenic mice.
hFcγRI conserves its properties as a high-affinity IgG receptor in transgenic mice. (A-B) Representative histogram plots of hFcγRI expression on indicated cell populations from blood or tissues of hFcγRItg 5KO mice (A) or blood of normal human donors (B). Two representative histogram plots from 2 different donors (#1 and #2) are represented for hFcγRI expression on neutrophils. (C) Histograms showing the expression of the respective FcγRs (FLAG) or the binding of indicated mouse monomeric IgG to FLAG-tagged FcγR+ CHO transfectants. Solid gray histograms represent the binding of secondary antibodies alone. (D) Histograms show the expression of the respective FcγRs (FLAG) or the binding of indicated IgG ICs (black line) or Ag alone (solid gray histograms) to FcγR+ CHO transfectants, as revealed by neutravidin staining. Note: the use of different secondary reagents to detect monomeric IgG (C) or IC (D) binding prevents comparing fluorescence intensities between histograms in panels C and D. (E-F) Real-time SPR sensorgrams and affinity constants were determined from SPR analysis. (E) Data correspond to the injection of 125nM hIgG1 (black) or mIgG2a (gray) onto immobilized hFcγRI. (F) Kinetic parameters determined from experiments presented in panel E and in supplemental Figure 1B. Data are representative of at least 2 independent experiments.
hFcγRI conserves its properties as a high-affinity IgG receptor in transgenic mice. (A-B) Representative histogram plots of hFcγRI expression on indicated cell populations from blood or tissues of hFcγRItg 5KO mice (A) or blood of normal human donors (B). Two representative histogram plots from 2 different donors (#1 and #2) are represented for hFcγRI expression on neutrophils. (C) Histograms showing the expression of the respective FcγRs (FLAG) or the binding of indicated mouse monomeric IgG to FLAG-tagged FcγR+ CHO transfectants. Solid gray histograms represent the binding of secondary antibodies alone. (D) Histograms show the expression of the respective FcγRs (FLAG) or the binding of indicated IgG ICs (black line) or Ag alone (solid gray histograms) to FcγR+ CHO transfectants, as revealed by neutravidin staining. Note: the use of different secondary reagents to detect monomeric IgG (C) or IC (D) binding prevents comparing fluorescence intensities between histograms in panels C and D. (E-F) Real-time SPR sensorgrams and affinity constants were determined from SPR analysis. (E) Data correspond to the injection of 125nM hIgG1 (black) or mIgG2a (gray) onto immobilized hFcγRI. (F) Kinetic parameters determined from experiments presented in panel E and in supplemental Figure 1B. Data are representative of at least 2 independent experiments.
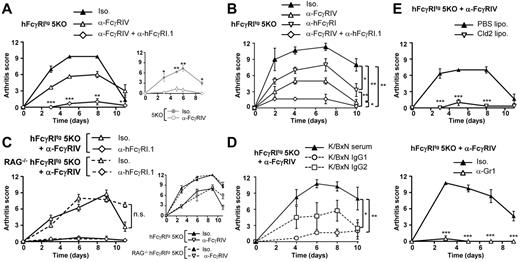
Because hFcγRI has been reported to be expressed in the articular synovium of arthritis patients, but not in healthy controls,31 we investigated whether hFcγRI could induce arthritic inflammation using hFcγRItg 5KO mice and K/BxN serum. The serum of spontaneously arthritic K/BxN mice (F1 offsprings from KRNtg mice crossed with NOD mice) indeed contained pathogenic IgG1 and IgG2 anti-GPI antibodies17 that were able to form ICs with GPI deposited on the articular cartilage. These ICs induce inflammatory arthritis that requires activating FcγRs.19 Both 5KO and hFcγRItg 5KO mice developed arthritis (Figure 2A) after K/BxN serum injection (K/BxN PA). Blocking FcγRIV using blocking anti-FcγRIV mAbs abolished arthritis in 5KO, but not in hFcγRItg 5KO mice. Blocking FcγRIV using anti-FcγRIV mAbs and hFcγRI using blocking anti-hFcγRI.1 mAbs (supplemental Figure 1C) was necessary to abolish K/BxN PA in hFcγRItg 5KO mice (Figure 2A). Blocking hFcγRI significantly reduced arthritis symptoms in hFcγRItg 5KO mice (Figure 2B). hFcγRI-dependent arthritis (ie, arthritis developing in anti-FcγRIV–treated hFcγRItg 5KO mice) was milder than arthritis developing in untreated hFcγRItg 5KO mice. Occupancy of a proportion of this human high-affinity receptor by endogenous mouse IgG may be responsible for these mild arthritic symptoms. hFcγRI-dependent arthritis did not, however, increase in severity when induced in RAG-deficient hFcγRItg 5KO mice that lack endogenous IgG (Figure 2C). Similar results were obtained for FcγRIV-dependent arthritis (Figure 2C inset). If occurring in vivo, partial occupancy or saturation of hFcγRI (or FcγRIV) by IgG therefore does not affect K/BxN arthritis induction and development. As expected, IgG2 antibodies purified from K/BxN serum induced hFcγRI-dependent arthritis, whereas IgG1 antibodies purified from K/BxN serum induced only very modest pathologic symptoms (Figure 2D). Finally, hFcγRI-dependent arthritis was abolished when monocytes/macrophages or neutrophils were depleted (Figure 2E). These results demonstrate that hFcγRI is sufficient to induce K/BxN passive arthritis that is mediated by mouse IgG2 autoantibodies and requires both monocytes/macrophages and neutrophils.
hFcγRI can trigger inflammatory arthritis in transgenic mice. (A-C) K/BxN PA in indicated mice injected with indicated mAbs (A-B, n = 3; C, n = 4). (D) Arthritis induced in anti-FcγRIV–treated hFcγRItg 5KO mice by K/BxN serum (n = 4) or 80 μg of purified K/BxN IgG1 (n = 3) or of purified K/BxN IgG2 (n = 4). (E) K/BxN PA in anti-FcγRIV–treated hFcγRItg 5KO mice injected with indicated liposomes (n = 3) or mAbs (n = 4). Data are representative of at least 2 independent experiments and are represented as means ± SEM. Statistical differences between curves (B-D) or for each time point (A,E) are indicated (for panel A, between the anti-FcγRIV– and the anti-FcγRIV + anti-hFcγRI.1–treated groups).
hFcγRI can trigger inflammatory arthritis in transgenic mice. (A-C) K/BxN PA in indicated mice injected with indicated mAbs (A-B, n = 3; C, n = 4). (D) Arthritis induced in anti-FcγRIV–treated hFcγRItg 5KO mice by K/BxN serum (n = 4) or 80 μg of purified K/BxN IgG1 (n = 3) or of purified K/BxN IgG2 (n = 4). (E) K/BxN PA in anti-FcγRIV–treated hFcγRItg 5KO mice injected with indicated liposomes (n = 3) or mAbs (n = 4). Data are representative of at least 2 independent experiments and are represented as means ± SEM. Statistical differences between curves (B-D) or for each time point (A,E) are indicated (for panel A, between the anti-FcγRIV– and the anti-FcγRIV + anti-hFcγRI.1–treated groups).
hFcγRI can trigger antibody-dependent airway inflammation
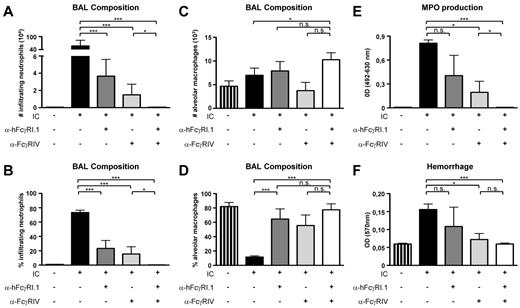
We next investigated whether hFcγRI could induce lung inflammation in a model of IC-mediated airway inflammation28 because hFcγRI is expressed on lung and alveolar macrophages from hFcγRItg 5KO mice (Figure 1A). This disease model of a reverse Arthus reaction consists of an IV injection of antigen (OVA) and intranasal instillation of anti-OVA antibodies and was shown to depend on the expression of activating FcRs on alveolar macrophages.26 IV injection of OVA followed by intranasal instillation of rabbit anti-OVA serum (hFcγRI binds rabbit IgG, supplemental Figure 1D) led to a massive infiltration of neutrophils in the airways within 18 hours, as determined in BALs. Whereas blocking either hFcγRI or mFcγRIV significantly inhibited neutrophil infiltration, blocking both hFcγRI and FcγRIV was necessary to abolish neutrophil infiltration (Figure 3A-B). No major variations in alveolar macrophage numbers under these different conditions were observed (Figure 3C), as expected.28 However, when they did occur, neutrophil infiltration drastically modified the alveolar macrophage/neutrophil ratio in the BAL (Figure 3D vs B). Similarly, whereas myeloperoxidase production in the BAL (Figure 3E) resulting from neutrophil and/or macrophage activation and hemorrhage (Figure 3F) resulting from tissue damage had a trend of being reduced after hFcγRI blockade and was significantly reduced after mFcγRIV blockade, both symptoms were abolished after blockage of both receptors. These results demonstrate that hFcγRI is sufficient to induce airway inflammation.
hFcγRI can trigger IC-induced airway inflammation in transgenic mice. (A-B) Neutrophil count (A) and percentage (B) among leukocytes. (C-D) Alveolar macrophage count (C) and percentage (D) among leukocytes. (E-F) Myeloperoxidase (MPO; E) level and hemorrhage score (F) in BAL from hFcγRItg 5KO mice after injection of the indicated reagents. IC indicates OVA injected intravenously followed by anti-OVA antiserum injected intranasally (n = 4 in all groups). Data are representative of at least 2 independent experiments and are represented as means ± SEM.
hFcγRI can trigger IC-induced airway inflammation in transgenic mice. (A-B) Neutrophil count (A) and percentage (B) among leukocytes. (C-D) Alveolar macrophage count (C) and percentage (D) among leukocytes. (E-F) Myeloperoxidase (MPO; E) level and hemorrhage score (F) in BAL from hFcγRItg 5KO mice after injection of the indicated reagents. IC indicates OVA injected intravenously followed by anti-OVA antiserum injected intranasally (n = 4 in all groups). Data are representative of at least 2 independent experiments and are represented as means ± SEM.
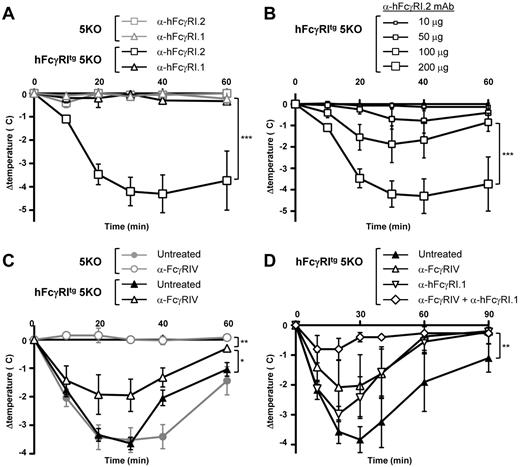
hFcγRI can trigger PSA
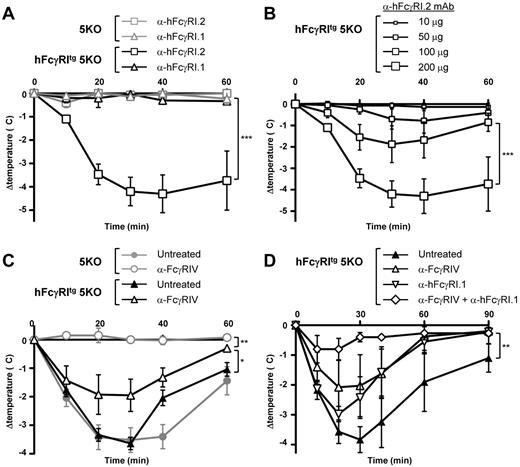
We reported recently that FcγRIV was responsible for IgG2b-induced PSA21 that arises after IV injection of preformed ICs made of mouse IgG2b (anti-DNP) and antigen (DNP-BSA). We therefore investigated the potential of hFcγRI, which has the same expression pattern and ligands as FcγRIV in transgenic mice, to induce PSA in hFcγRItg 5KO mice using divalent (anti-hFcγRI mAbs) or multivalent (IgG-IC) ligands. An IV injection of the nonblocking anti-hFcγRI.2 mAb, but not of the blocking anti-hFcγRI.1 mAb (supplemental Figure 1C), induced a significant temperature decrease in hFcγRItg 5KO mice, but not in 5KO mice (Figure 4A). The effect of nonblocking anti-hFcγRI.2 mAb injections on the central temperature of hFcγRItg 5KO mice was dose dependent (Figure 4B) and resulted in fatal anaphylactic shocks at higher doses (data not shown). Therefore, whereas anti-hFcγRI.1 mAb is an antagonistic blocking antibody, anti-hFcγRI.2 mAb is an agonistic nonblocking antibody capable of inducing hFcγRI-dependent anaphylaxis. In all further experiments, the in vivo hFcγRI blockade was achieved by anti-hFcγRI.1 mAb injections. An IV injection of mouse IgG2b-ICs induced a temperature decrease in 5KO and hFcγRItg 5KO mice that was abolished by FcγRIV blockade in 5KO mice, as expected,21 but not in hFcγRItg 5KO mice (Figure 4C). Confirming the anaphylactogenic potential of hFcγRI, blocking hFcγRI reduced the temperature decrease in hFcγRItg 5KO mice, and hFcγRI-dependent PSA (anaphylaxis developing in anti-FcγRIV–treated hFcγRItg 5KO mice) was abrogated by hFcγRI blockade (Figure 4D). These results demonstrate that hFcγRI is sufficient to trigger PSA in transgenic mice.
In vivo aggregation of hFcγRI induces PSA. (A-B) Indicated mice were injected with 200 μg of anti-hFcγRI.1 blocking mAb or anti-hFcγRI.2 nonblocking mAb (A) or with the indicated amounts of anti-hFcγRI.2 nonblocking mAb (B), and central temperatures were monitored (n ≥ 3). The same curve corresponding to 200 μg of anti-hFcγRI.2 nonblocking mAb injected in hFcγRItg 5KO mice is represented in experiments shown in panels A and B, which were performed together. Note: anti-hFcγRI.1 mAb is an antagonistic blocking antibody and anti-hFcγRI.2 mAb an agonistic nonblocking antibody. (C-D) 5KO and/or hFcγRItg 5KO mice were pretreated with indicated reagents and injected with preformed mouse IC made of mouse polyclonal anti-GPI serum and GPI, and central temperatures were monitored (C, n ≥ 4; D, n ≥ 3). Data are representative of at least 2 independent experiments and are represented as means ± SEM (for panel D, between the untreated and the anti-FcγRIV + anti-hFcγRI.1–treated groups).
In vivo aggregation of hFcγRI induces PSA. (A-B) Indicated mice were injected with 200 μg of anti-hFcγRI.1 blocking mAb or anti-hFcγRI.2 nonblocking mAb (A) or with the indicated amounts of anti-hFcγRI.2 nonblocking mAb (B), and central temperatures were monitored (n ≥ 3). The same curve corresponding to 200 μg of anti-hFcγRI.2 nonblocking mAb injected in hFcγRItg 5KO mice is represented in experiments shown in panels A and B, which were performed together. Note: anti-hFcγRI.1 mAb is an antagonistic blocking antibody and anti-hFcγRI.2 mAb an agonistic nonblocking antibody. (C-D) 5KO and/or hFcγRItg 5KO mice were pretreated with indicated reagents and injected with preformed mouse IC made of mouse polyclonal anti-GPI serum and GPI, and central temperatures were monitored (C, n ≥ 4; D, n ≥ 3). Data are representative of at least 2 independent experiments and are represented as means ± SEM (for panel D, between the untreated and the anti-FcγRIV + anti-hFcγRI.1–treated groups).
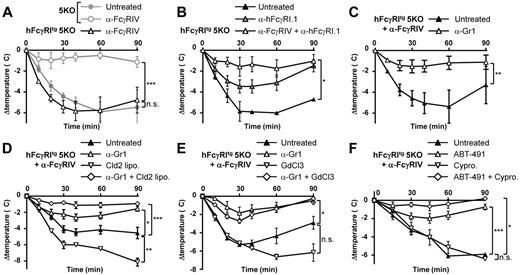
Neutrophils and PAF mediate hFcγRI-dependent ASA
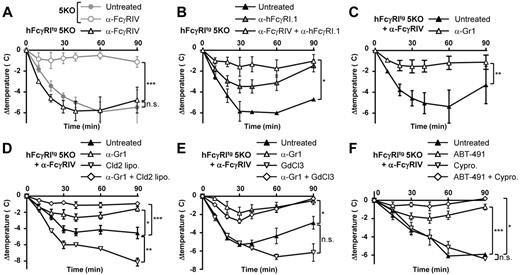
Because hFcγRI was sufficient to trigger PSA, we investigated whether hFcγRI may also trigger ASA. ASA was induced by an IV antigen (BSA) challenge in mice repeatedly immunized with the same antigen in Freund adjuvant (first immunization in complete and second and third immunizations in incomplete Freund adjuvant). This protocol induced a strong body temperature decrease in hFcγRItg 5KO mice, but not in 5KO mice, when pretreated with anti-FcγRIV mAbs (Figure 5A). We called this hFcγRI-dependent ASA. Supporting this result, hFcγRI blockade significantly inhibited the ASA-induced temperature decrease (Figure 5B) and abolished ASA-induced mortality (supplemental Figure 2A) in hFcγRItg 5KO mice. Blocking both hFcγRI and FcγRIV further inhibited ASA-induced temperature decreases in these mice (Figure 5B). Therefore, hFcγRI is sufficient to trigger ASA in transgenic mice.
Neutrophils are necessary for hFcγRI-dependent ASA. Mice were immunized with BSA in Freund adjuvant and challenged with BSA and their central temperatures and survival rates were monitored. (A-B) ASA in hFcγRItg 5KO and/or 5KO mice injected with indicated reagents (n = 5). (C-F) ASA in anti-FcγRIV–treated hFcγRItg 5KO mice injected with indicated reagents (C, n ≥ 4; D, n = 5; E, n = 5; F, n ≥ 3). Data are representative from at least 2 independent experiments and are represented as means ± SEM. Cld2 lipo indicates toxic liposomes; GdCl2, gadolinium; and Cypro, cyproheptadine.
Neutrophils are necessary for hFcγRI-dependent ASA. Mice were immunized with BSA in Freund adjuvant and challenged with BSA and their central temperatures and survival rates were monitored. (A-B) ASA in hFcγRItg 5KO and/or 5KO mice injected with indicated reagents (n = 5). (C-F) ASA in anti-FcγRIV–treated hFcγRItg 5KO mice injected with indicated reagents (C, n ≥ 4; D, n = 5; E, n = 5; F, n ≥ 3). Data are representative from at least 2 independent experiments and are represented as means ± SEM. Cld2 lipo indicates toxic liposomes; GdCl2, gadolinium; and Cypro, cyproheptadine.
Both effector cell types that express hFcγRI, monocytes/macrophages32 and neutrophils,21 can potentially contribute to ASA. hFcγRI-dependent ASA was strongly inhibited by neutrophil depletion after injection of anti-Gr1 mAbs (Figure 5C). Because this rat IgG2b anti-Gr1 mAb injection may lead to activation and depletion of complement components due to in vivo IC formation, as suggested previously,33 we investigated whether the inhibition of hFcγRI-mediated active anaphylaxis after anti-Gr1 mAb treatment relied on complement. A dose of cobra venom factor (CVF) that inactivates both C3 and C5 components of the complement34 did neither prevent hFcγRI-mediated active anaphylaxis nor its inhibition after anti-Gr1 mAb injections (supplemental Figure 2B). Therefore, the inhibition of anaphylaxis after anti-Gr1 mAb injection is dependent on neutrophil depletion per se and not on complement. Surprisingly, neither monocyte/macrophage depletion after toxic liposome injection (Figure 5D) nor inhibition of monocyte/macrophage function after gadolinium injection (Figure 5E) reduced hFcγRI-dependent ASA. Unexpectedly, the injection of toxic liposomes or gadolinium increased hFcγRI-induced hypothermia. However, the depletion or inhibition of monocytes/macrophages, when combined with the depletion of neutrophils, had a tendency to increase the protection from hFcγRI-dependent ASA (Figure 5D-E). Neutrophils and, possibly to a minor extent, monocytes/macrophages therefore contribute to hFcγRI-dependent ASA. Mediators released and/or secreted by these activated cell types should therefore be responsible for the anaphylactic shock observed. Among them, platelet activating factor (PAF) was shown to be responsible for neutrophil-dependent ASA21 and for macrophage-dependent ASA,32 whereas histamine was shown to be responsible for mast cell–dependent anaphylaxis.35 The PAF-R antagonist ABT-491, but not the histamine and serotonin receptor antagonist cyproheptadine, markedly reduced hFcγRI-dependent temperature decrease (Figure 5F) and mortality (supplemental Figure 2C). PAF therefore accounts for hFcγRI-dependent ASA. However, the conjunction of both antagonists further reduced hFcγRI-dependent ASA (Figure 5F). In addition to mast cells and basophils, neutrophils have been reported to be able to release histamine36 but not serotonin, suggesting that histamine released by neutrophils might, to a minor extent, contribute to hFcγRI-dependent ASA.
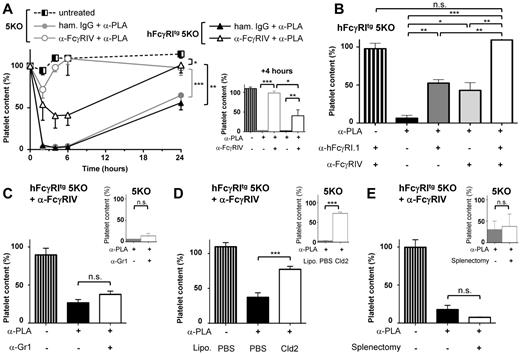
Monocytes/macrophages mediate hFcγRI-dependent thrombocytopenia
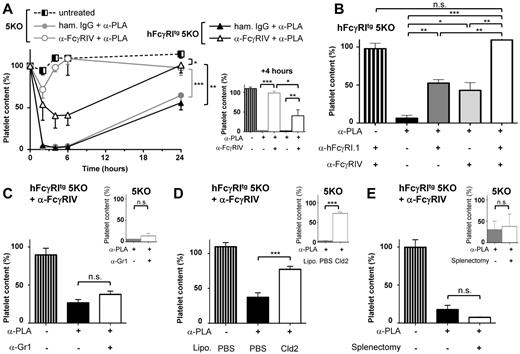
We next investigated whether, in addition to exerting pro-inflammatory and pro-anaphylactic properties, hFcγRI may also exert phagocytic properties in vivo using a murine model of thrombocytopenia. Immune thrombocytopenic purpura (ITP) can be induced by injecting IV antiplatelet antibodies (reminiscent of autoantibodies found in ITP patients) and by monitoring circulating platelet consumption. ITP could be induced after injection of mouse IgG2a antiplatelet mAbs both in hFcγRItg 5KO mice and in 5KO mice. FcγRIV blockade prevented ITP in 5KO mice (as expected based on previous findings21,22 ), but reduced platelet consumption by less than 50% in hFcγRItg 5KO mice (Figure 6A-B). The remaining platelet consumption was hFcγRI dependent, because it was prevented by a further hFcγRI blockade (Figure 6B). hFcγRI-dependent ITP was not affected by neutrophil depletion (Figure 6C), but was significantly inhibited by monocyte/macrophage depletion (Figure 6D). Splenectomy had no significant effect on hFcγRI-dependent ITP (Figure 6E), suggesting that hFcγRI-expressing macrophages other than splenic macrophages contribute to platelet clearance in this model. Liver macrophages (ie, Kupffer cells), which belong to the mononuclear phagocyte system, express hFcγRI in hFcγRItg 5KO mice (Figure 1A), could be responsible for platelet consumption in this model.
Macrophages are necessary for hFcγRI-dependent thrombocytopenia. (A) hFcγRItg 5KO (black) or 5KO (gray) mice were pretreated with the indicated reagents before being injected intravenously with antiplatelet mAb (α-PLA). Platelet counts were acquired in blood at the indicated times presented as curves (left) or at t = 4 hours presented as histograms (right) after α-PLA injection (n = 3). (B) hFcγRItg 5KO mice were pretreated with the indicated reagents and platelet counts acquired in the blood at t = 4 hours after α-PLA injection (n = 3). (C-E) 5KO mice (small histograms in insets) or anti-FcγRIV–treated hFcγRItg 5KO mice (large histograms, left in each panel) were pretreated with the indicated reagents or splenectomized when indicated and platelet counts were acquired in the blood at t = 4 hours after α-PLA injection (C-D: n = 3; E: n ≥ 3). Data are representative of at least 2 independent experiments and are represented as means ± SEM.
Macrophages are necessary for hFcγRI-dependent thrombocytopenia. (A) hFcγRItg 5KO (black) or 5KO (gray) mice were pretreated with the indicated reagents before being injected intravenously with antiplatelet mAb (α-PLA). Platelet counts were acquired in blood at the indicated times presented as curves (left) or at t = 4 hours presented as histograms (right) after α-PLA injection (n = 3). (B) hFcγRItg 5KO mice were pretreated with the indicated reagents and platelet counts acquired in the blood at t = 4 hours after α-PLA injection (n = 3). (C-E) 5KO mice (small histograms in insets) or anti-FcγRIV–treated hFcγRItg 5KO mice (large histograms, left in each panel) were pretreated with the indicated reagents or splenectomized when indicated and platelet counts were acquired in the blood at t = 4 hours after α-PLA injection (C-D: n = 3; E: n ≥ 3). Data are representative of at least 2 independent experiments and are represented as means ± SEM.
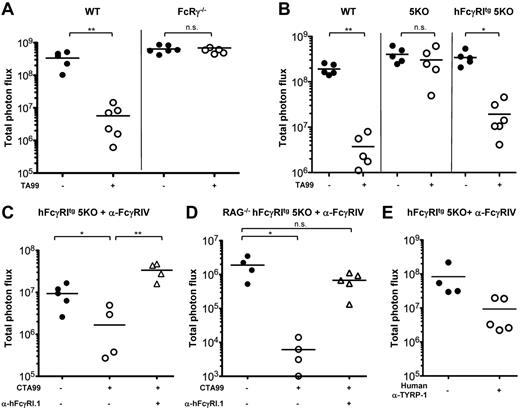
hFcγRI can mediate Ab-induced antitumor immunotherapy
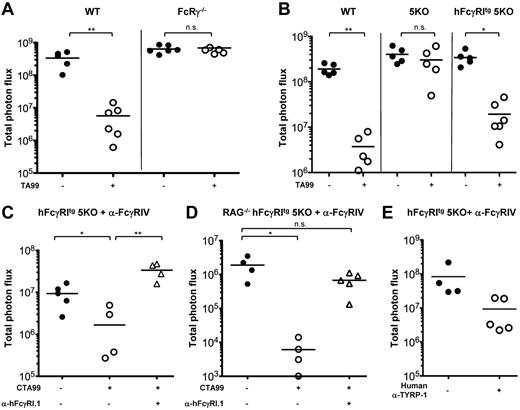
Because hFcγRI can mediate Ab-induced platelet clearance, we investigated whether hFcγRI may also mediate tumor reduction/destruction after antitumor Ab injection. We used the B16 melanoma (expressing gp75 aka TYRP-1) tumor immunotherapy model that relies on injections of anti–TYRP-1 mouse IgG2a TA99 mAb and that was reported to involve the contribution of the mouse homolog mFcγRI12,37,38 of hFcγRI. To allow accurate quantification of lung metastases (ie, tumor load) after IV injection of the tumor, we used a luciferase-expressing variant of B16 (B16 luc2+) that expresses similar amounts of TYRP-1 as wild-type (wt) B16 cells (supplemental Figure 3A). IV injections of B16 wt or B16 luc2+ cells in wt C57BL/6J mice led to metastatic melanoma in the lung that could be quantified by bioluminescence imaging on explanted lungs ex vivo in the case of B16 luc2+-injected mice (supplemental Figure 3B). Repeated TA99 injections lead to a drastic reduction in tumor load in wt C57BL/6J mice, but not in FcRγ−/− mice that lack all activating FcRs (Figure 7A) or in 5KO mice (Figure 7B). However, TA99 injections did lead to a significant reduction in tumor load in hFcγRItg 5KO mice (Figure 7B). Therefore, hFcγRI can mediate metastatic melanoma reduction after mouse IgG2a anti–TYRP-1 mAb injections.
hFcγRI can mediate antibody-dependent protection from metastatic melanoma in transgenic mice. (A-D) Mice were injected intravenously with B16 luc2+ cells and with anti–TYRP-1 mAbs (TA99 or CTA99 or human anti–TYRP-1) where indicated. (C-E) Mice were also pretreated with anti-FcγRIV mAbs. Quantification of tumor load was performed on ex vivo explanted lungs on day 11 after injection of B16 luc2+ cells (A, n ≥ 5; B, n ≥ 5; C, n ≥ 4; D, n ≥ 4; E, n ≥ 4). Data are representative of at least 2 independent experiments and are represented as means ± SEM.
hFcγRI can mediate antibody-dependent protection from metastatic melanoma in transgenic mice. (A-D) Mice were injected intravenously with B16 luc2+ cells and with anti–TYRP-1 mAbs (TA99 or CTA99 or human anti–TYRP-1) where indicated. (C-E) Mice were also pretreated with anti-FcγRIV mAbs. Quantification of tumor load was performed on ex vivo explanted lungs on day 11 after injection of B16 luc2+ cells (A, n ≥ 5; B, n ≥ 5; C, n ≥ 4; D, n ≥ 4; E, n ≥ 4). Data are representative of at least 2 independent experiments and are represented as means ± SEM.
A chimeric version of TA99 with a human IgG1 heavy chain (CTA99; developed by Imclone, US patent 2009/0232823 A1) has been constructed to test the therapeutic efficacy of this mAb in clinical trials. Heat aggregates of CTA99 (or human polyclonal IgG1) mimicking ICs readily bound hFcγRI in vitro (supplemental Figure 3C). CTA99 injections led to a significant reduction in tumor load in hFcγRItg 5KO mice pretreated with anti-FcγRIV mAbs that was abolished by hFcγRI blockade (Figure 7C). A significant reduction in tumor load after CTA99 injection and an abolition of this effect after hFcγRI blockade were also obtained in anti-FcγRIV mAb pretreated RAG-deficient hFcγRItg 5KO mice that cannot produce endogenous antibodies (Figure 7D). Furthermore, injections of a fully human IgG1 mAb anti–TYRP-139 had a tendency to reduce tumor loads in hFcγRItg 5KO mice pretreated with anti-FcγRIV mAbs (Figure 7E). Therefore, hFcγRI mediates Ab-induced reduction of tumor load in transgenic mice after injection of humanized anti–TYRP-1 mAbs.
Discussion
The results of the present study suggest that although hFcγRI is characterized as a high-affinity receptor for IgG, hFcγRI is readily available in vivo to bind IgG-ICs or IgG-opsonized targets. Despite its potential saturation by IgG in vivo, hFcγRI was indeed sufficient to mediate pro-inflammatory, pro-anaphylactic, and antitumor functions, leading to autoimmune, allergic, and therapeutic reactions, respectively, in transgenic mice. Neutrophils contributed predominantly to hFcγRI-induced anaphylaxis, whereas monocytes/macrophages contributed predominantly to hFcγRI-induced autoimmune thrombocytopenia. Both neutrophils and monocytes/macrophages were, however, required for hFcγRI-induced autoimmune arthritis, demonstrating their nonredundant roles in this arthritis model. hFcγRI-expressing resident macrophages may attract circulating hFcγRI-expressing neutrophils that are responsible for inflammation and cartilage destruction in this arthritis model, as suggested from studies using wt mice.27,29
To investigate the role of human FcγRI in vivo, we used transgenic mice for this receptor30 that display an expression pattern of hFcγRI comparable to that found in humans. Monocytes, macrophages, and DCs in humans and in these transgenic mice indeed express hFcγRI. However, hFcγRI was reported to be inducible on human neutrophils, whereas neutrophils from hFcγRItg mice constitutively express hFcγRI. Nevertheless, hFcγRI was reported to be expressed on human neutrophils under multiple circumstances, including in particular rheumatoid arthritis23 and multiple myeloma.25 One can therefore consider that human neutrophils may express hFcγRI in most inflammatory contexts. To avoid a possible in vivo competition or contribution of endogenous FcγRs to reactions mediated by hFcγRI, we crossed hFcγRI-transgenic mice with 5KO mice that lack FcγRI, FcγRIIB, FcγRIII, FcϵRI, and FcϵRII.7 The resulting hFcγRItg 5KO mice express only 2 activating FcRs, transgenic hFcγRI and endogenous FcγRIV, which could be efficiently blocked in vivo to study the specific contribution of hFcγRI to a particular disease or therapy model. The expression of the transgene in this background led to an increased expression level of hFcγRI on neutrophils in transgenic mice compared with humans, but a very similar expression on monocytes. Testing anti-hFcγRI–specific mAbs in vivo in these mice revealed an agonist/nonblocking activity (anti-hFcγRI.2 mAb) or an antagonist/blocking activity (anti-hFcγRI.1 mAb). hFcγRI bound not only human IgG1/3/4 subclasses,6 but also mouse IgG2a/2b subclasses as monomers. The affinity of hFcγRI for mIgG2a was very similar to its affinity for hIgG1 (KD ∼ 38nM and 40nM, respectively), in the range of the high-affinity mIgG2a-mFcγRIV interaction (KD ∼ 34nM).22 Therefore, hFcγRI functions as a high-affinity IgG receptor not only in humans, but also in hFcγRItg mice. The fact that hFcγRI conserved its high-affinity properties for mouse IgG validates hFcγRItg mice as a model with which to study the contribution of hFcγRI to disease and therapy.
In hFcγRItg mice, we found that the engagement of hFcγRI alone or of FcγRIV alone resulted in reactions with a lower intensity than the engagement of both receptors. Because hFcγRI and FcγRIV associate with the same FcRγ-subunit to mediate signal transduction, their aggregation by ICs should not lead to qualitatively different responses. Insufficient expression levels or occupancy of a proportion of these high-affinity receptors by endogenous (monomeric) IgG2 may, however, explain this phenomenon. The latter possibility, described previously,40 certainly dissuaded many from investigating the role of hFcγRI in IgG-mediated effector reactions in vivo. In the present study, we demonstrate that hFcγRI can readily induce inflammatory reactions after passive administration of pathogenic IgG despite its ability to be bound/saturated by endogenous monomeric IgG. In addition, the intensity and kinetics of the responses triggered by hFcγRI were comparable to those triggered by low-affinity FcRs. Supporting our observations, the mouse high-affinity FcRs FcγRI and FcγRIV were reported to play similar roles as mouse low-affinity FcγRIII in models of inflammation.19,21,28 Finally, we observed no difference in the kinetics of the appearance of hFcγRI-dependent arthritic symptoms, nor in their severity, between IgG-sufficient (hFcγRItg 5KO) and IgG-deficient (RAG-deficient hFcγRItg 5KO) mice.
This work and previous studies support the notion that being of high or low affinity for IgG, FcγRs engaged by a given multivalent ligand and expressed by a given cell will induce with comparable kinetics the activation of that cell and consequently in vivo responses. It follows that the ability of high-affinity FcγRs to bind monomeric IgG has no detectable consequence in vivo. One could therefore consider that high-affinity FcγRs remain as unoccupied as low-affinity FcγRs in vivo. Nevertheless, the high concentration of circulating IgG favors the hypothesis that at any given time a proportion of high-affinity, but also low-affinity, FcγRs are interacting with IgG. Low- and high-affinity FcγRs were indeed reported to bind monomeric IgG with a half-life of the interaction varying from less than 1 minute to more than 10 minutes,7,20,22,41 respectively. Consistent with these previous results, we report herein an approximately 4-minute half-life for the interaction of hFcγRI with hIgG1 or with mIgG2a. Results obtained in vivo nevertheless suggest that these half-lives are sufficiently short to allow low- and high-affinity FcγRs to rapidly bind IgG-ICs and to induce cell activation.
We found that hFcγRI can induce several allergy-related reactions in hFcγRItg mice. In the model of airway inflammation, hFcγRI triggered neutrophil infiltration, hemorrhage, and myeloperoxidase production in the alveolar space, symptoms that are reminiscent of those found in asthma patients. Whereas this model has been reported to be macrophage dependent, we could not formally demonstrate the contribution of these cells to hFcγRI-induced airway inflammation because of inefficient depletion of alveolar macrophages. Nevertheless, the fact that alveolar macrophages represent more than 90%-95% of the cells in the BAL of unchallenged mice and that they express hFcγRI supports a role for alveolar macrophages in this reaction. hFcγRI was also able to induce PSA when triggered by divalent or multivalent ligands and by ASA. As with ASA in wt mice,21 hFcγRI-induced ASA relied predominantly on neutrophils and PAF. Because hFcγRI is expressed at higher levels on neutrophils from hFcγRItg mice than on those from humans, the contribution of neutrophils might be overestimated in the mouse model we used. The expression of hFcγRI is, nevertheless, not higher on neutrophils than on monocytes and macrophages in these mice. Surprisingly, whereas monocytes/macrophages were reported to contribute predominantly to human FcγRIIA-induced systemic anaphylaxis2 and to particular models of passive and active anaphylaxis,32 monocytes/macrophages did not significantly contribute to anaphylaxis in hFcγRItg mice. Whereas it has been reported that hFcγRI is expressed on in vitro–stimulated human cord blood–derived mast cells,42 it has not been reported on human skin mast cells43 or mast cells from hFcγRItg mice (present study). Whatever the relative contribution of these cell subsets to allergic and anaphylactic reactions in humans, our results suggest that hFcγRI may be a key player in allergic and anaphylactic reactions in humans when allergen-specific IgGs are present.
hFcγRI has been reported to allow antigen targeting to DCs to enhance antigen presentation,24 and we report herein that hFcγRI contributes to the induction of several inflammatory models in hFcγRItg mice. The mouse homolog of FcγRI, mFcγRI, is also expressed on DC populations, particularly monocyte-derived DCs,16,18 and has been reported to play similar roles as hFcγRI in enhancing antigen presentation of IgG-bound antigen.11 Whereas the expression of mFcγRI on circulating monocytes and macrophage subsets is under debate,10,11,15-17 expression of mFcγRI could not be detected on neutrophils in steady-state conditions, during inflammatory arthritis, or in tumor-bearing mice (data not shown). The absence of mFcγRI on these effector cells suggests that one of its main activities may be to favor antigen presentation by and activation of DCs, in agreement with its contributions reported after active immunization protocols.10,11 Passive models of disease using mFcγRI−/− mice nevertheless reported an effect of mFcγRI deficiency in IC-induced Arthus reactions in the footpad11 and in Ab-induced autoimmune hemolytic anemia.10,13 mFcγRI may therefore be a functional homolog of hFcγRI when considering DCs. When considering neutrophils, however, mFcγRIV, which does not exist in humans, may be a functional homolog of hFcγRI. Like hFcγRI (present study), mFcγRIV is indeed expressed on these cell subsets7,22 and was reported to contribute to anaphylaxis,21 arthritis,17 airway inflammation,26 and thrombocytopenia.20,21 We therefore propose that hFcγRI may recapitulate in humans the roles played in mice by mFcγRI on DCs to favor antigen presentation and cell activation and by mFcγRIV on neutrophils to trigger effector (pro-inflammatory) reactions. Whether mFcγRI and/or mFcγRIV recapitulates in mice the roles played by hFcγRI in humans is unclear and will require more investigation, in particular in the expression of mFcγRI on monocyte/macrophage subpopulations.
The model of B16 metastatic melanoma has been used extensively to study the contribution of FcRs to experimental antibody-based immunotherapy. Using a bioluminescent variant of B16 and either the mouse IgG2a anti–TYRP-1 mAb TA99 or its humanized version CTA99 bearing the constant regions of a human IgG1, we report herein that hFcγRI can mediate antibody-based immunotherapy. hFcγRI may thus contribute to (or be responsible for) the reduction of B16 metastatic melanoma recently observed in mice expressing multiple hFcγRs injected with a humanized anti–TYRP-1 mAb TA99.44 Furthermore, we demonstrate in the present study that hFcγRI could mediate the protective effect of a fully human IgG1 anti–TYRP-1 mAb, which is currently being evaluated in a phase 1 trial involving patients suffering from malignant melanoma. CTA99 and fully human anti–TYRP-1 were, however, less efficient than TA99 in the mouse model of metastatic melanoma. In mice and humans, the neonatal IgG receptor FcRn is responsible for the protection of IgG degradation and contributes to IgG distribution into tissues.45 The binding of human IgG1 to mouse FcRn is almost 3 times lower than the binding of mouse IgG2a to mouse FcRn.46 Therefore, the half-life and/or biodistribution of human IgG1 may be reduced compared with that of mouse IgG2a when injected in mice, suggesting a reduced opsonization and elimination of tumor cells. The mechanism by which hFcγRI mediates the protective effect of anti–TYRP-1 mouse mAb TA99, humanized IgG1 mAb CTA99, and fully human IgG1 mAb on metastatic melanoma remains to be identified, but should not require NK cells because these cells do not express hFcγRI. However, myeloid cells, macrophages in particular, might be responsible for metastasis reduction in this model. Intriguingly, in the absence of all other FcγRs, mFcγRIV was not sufficient to mediate TA99-based tumor immunotherapy, whereas its absence (FcγRIV−/− mice) or in vivo blockade in wt mice (anti-FcγRIV mAbs) has been reported to reduce the efficiency of TA99 in this model.20,47 The expression of hFcγRI was sufficient to restore antibody-based tumor immunotherapy in mice that could not mediate this property anymore. This property of hFcγRI is reminiscent of that found for its mouse homolog mFcγRI in mediating the protective effects of anti–TYRP-1 mAb TA99 on B16 lung metastases38 or liver metastases.37
Most current preclinical studies based on hFcγRI only exploit its functions in favoring antigen presentation.48-50 In addition to these properties, we report in the present study that hFcγRI can also mediate the protective effects of antitumor antibodies on melanoma metastases and therefore potentially also on solid tumors. Supporting this assumption, bispecific antibodies directed against hFcγRI and c-erbB-2, a transmembrane receptor highly expressed in several human malignancies, indeed trigger hFcγRI-dependent antibody-dependent cell cytotoxicity in vitro.48-50 We also report herein that hFcγRI can induce several mouse models of autoimmune and allergic reactions and can therefore be considered a potential pro-inflammatory and pro-anaphylactic activating IgG receptor in humans. Anti-hFcγRI–blocking mAbs prevented hFcγRI-dependent models of autoimmunity and allergy and may therefore be assessed for their efficiency in human pathologies. Finally, our results indicate that hFcγRI, and potentially other high-affinity FcRs, are either not occupied/saturated by IgG in vivo or, if they are, this comes without functional consequences on their ability to mediate antitumor activities and pro-inflammatory and pro-anaphylactic properties.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M.-A. Nicola and the Plate-Forme d'Imagerie Dynamique for help with the bioluminescence experiments; C. Detchepare for administrative help (Institut Pasteur, Paris); our colleagues for their generous gifts: J. Van de Winkel (University Medical Center Utrecht, Utrecht, The Netherlands), S. Verbeek (Leiden University Medical Center, Leiden, The Netherlands), J.-P. Kinet (Harvard Institute of Medicine, Boston, MA), M. Lamers (Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany), D. Mathis, C. Benoist (Harvard Medical School, Boston, MA), and Institut de Génétique et de Biologie Moléculaire et Cellulaire (Illkirch, France) for mice; R. Coffman (DNAX, Palo Alto, CA), R. Good (University of South Florida College of Medicine, Tampa, FL), B. Heyman (Uppsala Universitet, Uppsala, Sweden), N. Hogg (Cancer Research UK, London, United Kingdom), and J.V. Ravetch (Rockefeller University, New York, NY) for antibodies. Cl2MDP was a gift of Roche Diagnostics.
This work was supported by the Institut Pasteur, Inserm, the Agence Nationale de la Recherche (ARC; grant GENOPAT-09-GENO-014-01), the Fondation ARC pour la Recherche sur le Cancer and the Ligue Nationale contre le Cancer (Comité de Paris), the Société Française d'Allergologie (Soutien de la Recherche en Allergologie 2010), the Arthritis Fondation Courtin, and the Balsan company. M.A. is a scholar of the Pasteur Paris University International Doctoral Program. D.A.M. and F.J. were supported in part by a fellowship from the Institut Pasteur (Bourse Roux) and from the Fondation pour la Recherche Médicale, respectively.
Authorship
Contribution: D.A.M. performed all of the experiments except the tumor experiments, which were performed by M.A.; D.A.M., M.A., M.D., and P.B. analyzed and discussed the results; D.A.M. and P.B. wrote the manuscript; F.J. contributed to several experiments; B.I. genotyped the mice and produced the reagents; N.V.R. and X.K. provided the reagents; P.E. designed and analyzed the surface plasmon resonance experiments; and P.B. designed and supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Bruhns, Laboratoire Anticorps en Thérapie et Pathologie, Département d'Immunologie, Institut Pasteur, 25 rue du Docteur Roux, 75015 Paris, France; e-mail: bruhns@pasteur.fr.