Abstract
Patients with cancer have an increased risk for venous thrombosis. Interestingly, different cancer types have different rates of thrombosis, with pancreatic cancer having one of the highest rates. However, the mechanisms responsible for the increase in venous thrombosis in patients with cancer are not understood. Tissue factor (TF) is a transmembrane receptor and primary initiator of blood coagulation. Tumor cells express TF and spontaneously release TF-positive microparticles (MPs) into the blood. MPs are small membrane vesicles that are highly procoagulant. It has been proposed that these circulating tumor-derived, TF-positive MPs may explain the increased rates of venous thrombosis seen in patients with cancer. In animal models, increased levels of tumor-derived, TF-positive MPs are associated with activation of coagulation. Moreover, these MPs bind to sites of vascular injury and enhance thrombosis. We and others have found that patients with cancer have elevated levels of circulating TF-positive MPs. These MPs are derived from tumors because they express tumor markers and are decreased by tumor resection. Importantly, several studies have shown that increased levels of TF-positive MPs correlate with venous thrombosis in patients with cancer. Taken together, these results suggest that TF-positive MPs may be a useful biomarker to identify patients with cancer who are at high risk for thrombosis.
Introduction
Cancer and its treatment are frequently complicated by the development of venous thromboembolism (VTE),1,2 which is a collective term for deep vein thrombosis (DVT) and pulmonary embolism (PE). The development of VTE in patients with cancer is a condition included in the spectrum of prothrombotic conditions in cancer that are encompassed by Trousseau’s syndrome.1
Thrombosis increases the morbidity and mortality of patients with cancer.3-5 Pancreatic and brain cancer have the highest rates of VTE among all cancer types, with cumulative incidences reported at 5.3% to 26% and 1.6% to 26%, respectively.5-7 Hematologic malignancies are also known to have a high rate of VTE. Reported incidence values for VTE in certain types of lymphoma are as high as 59.5%, and for multiple myeloma they are as high as 58%, with highly prothrombotic chemotherapy regimens.8 In comparison, lung and colorectal cancers have lower rates of VTE (from 1.6% to 13.6% and from 3.1% to 10.2%, respectively), and breast and prostate cancer have the lowest rates (from 0.4% to 8.1% and from 0.5% to 1.3%, respectively).5-7 In fact, cancer type was used in the development of a risk model to predict the development of VTE in patients with cancer.9 The epidemiology and treatment of cancer-induced thrombosis is discussed in detail in 2 other reviews in this series: one by Cannegieter and colleagues,10 and another by Lee and colleagues.11 Tissue factor (TF) is a transmembrane receptor that binds plasma factor VII/VIIa and triggers blood coagulation after vascular injury and in various diseases.12-17 TF is expressed by many cancer cells, particularly in cancers of epithelial origin.18-20 Cancer cells also spontaneously release small membrane microparticles (MPs) bearing TF.21,22 An early study proposed that these MPs may explain the prothrombotic state associated with malignancy.23,24
In this review, we summarize studies analyzing the role of TF-positive, tumor-derived MPs (TMPs) in the activation of coagulation and thrombosis in mouse models, as well as the association between TF-positive MPs and the development of VTE in patients with cancer.
Development of a venous thrombus
Venous clots form on the surface of a largely intact vascular endothelium because of a combination of changes in the vessel wall, blood flow disturbances, and thrombogenic factors in the blood itself.2,12,25-28 The most common site for the development of venous thrombi is valve pockets.26,27,29-32 A number of different mouse models have been used to study thrombosis.33 In general, animal models of thrombosis that do not produce extensive damage to the vessel wall are better models of venous thrombosis than models that expose vessel wall TF. Studies with healthy mice have shown that leukocyte TF contributes to thrombosis in the inferior vena cava (IVC) stenosis model, whereas vessel wall TF drives thrombosis in the IVC ligation and electrolytic injury models.34-38 In small vessels, thrombosis has been shown to be dependent on TF expression by neutrophils, hematopoietic cell-derived TF-positive MPs, and vessel wall TF.36,37
MPs
Cells release 3 types of membrane vesicles: exosomes (50-100 nM), MPs (0.1-1 µm), and apoptotic bodies (1-3 µm).39 This review focuses on TF-positive MPs.
Cells undergoing apoptosis release apoptotic bodies.40 Exosomes are released from the cell by the fusion of intracellular multivesicular bodies with the plasma membrane.40 A recent study reported that cancer cells undergoing epithelial-to-mesenchymal transition release TF-positive exosomes.41 MPs are released from most cell types upon activation, and platelets are the major cellular source of MPs in blood. Indeed, MPs were originally described as “platelet dust.”2,42-44 Importantly, tumor cells spontaneously release MPs.21,22
MPs are formed by the outward blebbing of the plasma membrane and are subsequently released as small phospholipid vesicles after proteolytic cleavage of the cytoskeleton.40,42 There is selective packaging of surface proteins into MPs,40 including TF12,21,45 and adhesion molecules.46,47 MPs are procoagulant because of the presence of TF2,12 and the exposure of negatively charged phospholipids, such as phosphatidylserine (PS).48 The negatively charged surface facilitates the assembly of positively charged coagulation protein complexes.13,46,49
Methods for the quantification of MPs
Several methods have been used for the quantification of TF-positive MPs in human plasma, including antigen- and activity-based assays.50,51 We believe that functional assays are the best way to measure TF in MPs because they are the most sensitive methods for detecting very low levels of active TF in plasma. In addition, the specificity of some TF antigen-based assays has been questioned.52-54 One study failed to observe a correlation between MP TF activity and 2 antigen-based assays (flow cytometry and a commercial TF enzyme-linked immunosorbent assay).55 In contrast, our laboratory found a strong correlation between MP TF activity and plasma TF protein levels using an “in-house” human TF enzyme-linked immunosorbent assay.56 Several functional assays have been developed that measure TF activity in isolated MPs, using either a single-stage clotting assay or a 2-stage factor Xa generation assay in the presence or absence of an inhibitory anti-TF antibody.55-59 Thaler and colleagues measured levels of MP TF activity in patients with cancer, and found a good correlation between 2 assays.60 We observed comparable levels of TF activity in MPs isolated from lipopolysaccharide-stimulated human whole blood, using 2 centrifugation speeds (either 20 000 × g for 20 minutes [primarily MPs] or 100 000 × g for 1 hour [MPs and exosomes]), suggesting that the majority of vesicle TF is on MPs.59
Light-scatter flow cytometry is the most commonly used method for the quantification of MPs in clinical samples.61 Several studies have used flow cytometry to detect TF-positive MPs in patients with cancer.55,62,63 However, the low sensitivity of flow cytometry makes it difficult to reliably detect the low levels of TF-positive MPs in clinical samples. A recent publication reported that the level of TF-positive MPs measured using a functional MP TF activity assay correlated with the development of VTE in patients with cancer, whereas no correlation was found using flow cytometry to measure TF-positive MPs.64 Interestingly, we failed to detect TF-positive MPs by flow cytometry in samples from LPS-treated whole blood that had high levels of MP TF activity.59 Impedance-based flow cytometry is an alternative method for measuring levels of TF-positive MPs65 ; it has been used to detect TF-positive MPs in plasma from patients with cancer.66,67
TF-positive MPs in animal studies
Animal studies have found that TMPs are released from a variety of tumors in vivo.23,53,68 An early study found that guinea pigs bearing hepatocarcinoma tumors had increased procoagulant activity in cell-free ascites fluid that could be pelleted by ultracentrifugation. The presence of membrane vesicles less than 1 µm in size in this pellet was confirmed by electron microscopy.23 The procoagulant activity of the MPs released from these tumor cells in vitro was later determined to be TF-dependent.24 A more recent study found that severe combined immune deficiency mice bearing TF-expressing human colorectal tumors had increased levels of tumor-derived human TF protein in plasma.68 Further, circulating TF levels correlated with the size of the tumor in these mice.68 Circulating TF-positive TMPs have also been found to be associated with the activation of coagulation in mice.21,41,53 Specifically, increased circulating levels of human TF protein in nude mice bearing orthotopic human pancreatic tumors was associated with activation of coagulation, as monitored by plasma levels of thrombin-antithrombin (TAT) complex.21 In vitro studies showed that the plasma from tumor-bearing nude mice generated increased thrombin compared with control plasma and that this increase could be inhibited by an anti-human TF monoclonal antibody.21 A similar elevation in plasma TAT complex levels was seen in severe combined immune deficiency mice bearing TF-positive tumors formed by the human squamous cell carcinoma cell line A431.41 We found that inhibition of human TF with a species-specific monoclonal antibody reduced TAT levels in nude mice bearing an orthotopic tumor derived from human pancreatic adenocarcinoma cell line HPAF-II.53 However, the relative contribution of TF expression by the TMPs and that of the tumor cells themselves to the activation of coagulation could not be determined.53
Several studies have also shown that TMPs enhance the development of thrombosis in mice in vivo. Thomas and colleagues found that both endogenously generated and exogenously injected PANC02 TMPs, but not tumor cells, accumulated at the site of ferric chloride–induced mesenteric vessel injury and laser-induced cremaster arteriole injury.69 In addition, accumulation of endogenous TF-positive TMPs in these orthotopic PANC02 pancreatic tumor–bearing mice was shown to be P-selectin-dependent. Interestingly, this study found that both human and mouse pancreatic cell lines expressed P-selectin glycoprotein ligand 1.69 In contrast, we did not detect P-selectin glycoprotein ligand 1 expression by 4 different human pancreatic cell lines but found that they bound to an immobilized P-selectin-IgG chimera, which indicated that they expressed a P-selectin ligand (J.-G. Wang and N. Mackman, unpublished data, 2012). Injected exogenous TMPs enhanced thrombosis, and this enhancement was abolished with an anti-P-selectin antibody.69 We found that mice bearing human pancreatic tumors had increased thrombosis compared with control mice in a ferric chloride saphenous vein model.53 However, as discussed previously, this is not the best model for studying venous thrombosis.
The IVC stenosis model of venous thrombosis is particularly suited for the evaluation of the mechanisms of VTE initiation because thrombosis is triggered by endothelial cell activation and changes in blood flow, rather than denudation of the endothelium and vessel wall damage.35 It should be noted, however, that there is significant variability in thrombosis observed in the IVC stenosis model.35,53,70-72 One study reported increased incidence of thrombosis in this model in C57BL/6J mice bearing subcutaneous PANC02 tumors.73 In contrast, we did not observe an increase in IVC thrombosis in nude mice bearing TF-positive human HPAF-II pancreatic tumors.53 We did find that injection of exogenous TF-positive HPAF-II MPs enhanced thrombosis, although the amount of injected TMPs required to increase thrombosis was 40 times higher than that present in mice with HPAF-II tumors.53 At present, it is unclear the reasons for these different results, but it may be because of differences in experimental conditions, use of different mouse strains, tumor size, and species of the tumors (mouse vs human). Clearly, further studies are needed to understand how TF-positive TMPs contribute to thrombosis in different mouse models.
TF expression in cancer
Cancer cells are well known to express TF and release TF-positive MPs.18-21,69,74 In addition, TF expression increases with histologic grade in different cancer types, including pancreatic cancer.19,20,75 Two studies have reported a correlation between the level of TF in pancreatic and brain tumors and VTE.20,75 In addition to its proposed role in cancer-associated thrombosis, TF has been shown to be involved in tumor growth and metastasis.76,77
Similarly, TF-positive MPs released from cancer cells have been implicated in non-VTE-related cancer processes.58,78 Tesselaar and colleagues found that patients with advanced breast cancer, but not patients with early-stage cancer, had elevated levels of MP TF activity compared with healthy controls.58 Similarly, we did not detect elevated levels of MP TF activity in a group of 26 patients with early-stage breast cancer.79 Thaler and colleagues recently identified a correlation between increased MP TF activity and worsened cancer stage, grade, and survival in patients with metastatic nonresectable pancreatic cancer.78
Circulating TF-positive TMPs and activation of coagulation in patients with cancer
Numerous studies have analyzed levels of circulating TF-positive MPs in patients with cancer. Hron and colleagues used flow cytometry to detect increased levels of TF-positive MPs in patients with advanced colorectal cancer (n = 20) compared with those found in age-matched healthy control individuals.62 Levels of TF-positive MPs in the patients with cancer correlated with activation of coagulation, as determined by d-dimer levels.62 A study of patients with early-stage prostate cancer (n = 69) also observed increased MP TF activity in those with a modest correlation between MP TF and d-dimer levels.55 In patients with glioblastoma, plasma levels of TF-positive MPs, but not MP TF activity, correlated with d-dimer levels.80,81 An observational study of a single patient with giant cell lung carcinoma who had a high rate of thromboembolic events reported vesicle-associated plasma TF antigen levels that were 41 times higher than those seen in 16 healthy control indivduals.45
Hron and colleagues also found that most of the circulating TF-positive MPs in their patients with advanced colorectal cancer coexpressed platelet antigen CD41a, indicating they were derived from platelets.62 Platelets do not appear to synthesize TF but have been shown to bind monocyte-derived, TF-positive MPs.82-84 Recently, we found that TMPs bind to platelets (J. E. Geddings, W. Bergmeier, N. Mackman, unpublished data). This suggests that TF present on platelet-derived MPs may be a result of the binding of TF-positive TMPs to platelets and their reprocessing to form platelet-derived MPs. We found an increase in TF activity in combined platelet and MP samples from patients with cancer compared with healthy control individuals.85 Therefore, levels of TF activity in a combined platelet and MP sample may be a more accurate measure of thrombotic risk in patients with cancer compared with the TF activity of MPs alone.
Cancer chemotherapy and TF-positive MPs
Cancer chemotherapy is known to be associated with increased thrombosis.7,8 We found that treatment of the human monocytic cell line THP-1 with cytotoxic chemotherapy agents enhanced cellular TF activity without increasing TF expression by increasing cellular PS exposure.86 Further, these cells demonstrated increased release of TF-positive MPs.86 Increased PS expression or release of PS-positive MPs has also been observed with cytotoxic chemotherapy treatment of other cell types, including endothelial cells, red blood cells, and acute promyelocytic leukemia cells.87-90
Tesselaar and colleagues found that patients with cancer receiving chemotherapy do not have elevated plasma MP TF activity in comparison to untreated patients with cancer.91 We evaluated the effect of chemotherapy on MP TF activity in 26 patients with early-stage breast cancer.79 Plasma samples were analyzed at days 0, 2, and 8 after the start of chemotherapy, during the first 2 cycles of chemotherapy, but no increase in MP TF activity was observed.79 A recent study suggested that the activation of coagulation in patients with early-stage breast cancer undergoing chemotherapy may be mediated by the release of free DNA.92
These findings suggest that mechanisms other than increased levels of circulating TF-positive MPs are responsible for thrombosis during cancer chemotherapy, such as release of nucleic acids and increased cellular PS exposure.
TF-positive TMPs and VTE in patients with cancer
Retrospective studies
The earliest retrospective study that analyzed the link between TF-positive MPs and VTE in patients with cancer was performed by Tesselaar and colleagues in 2007 (Table 1).58 This study included 23 patients with nonresectable pancreatic adenocarcinoma, 27 patients with breast ductal adenocarcinoma (10 patients with early- and 17 patients with late-stage breast cancer), 7 patients with idiopathic VTE, and 37 healthy control participants. Mean MP TF activity was significantly higher in patients with pancreatic and metastatic breast cancer compared with either healthy control participants or patients with idiopathic VTE. Interestingly, patients with pancreatic cancer had higher MP TF activity than patients with breast cancer. Patients with cancer who also had VTE had higher plasma MP TF activity than patients with cancer without VTE.58 Approximately 50% of MPs in the patients with cancer expressed the tumor antigen mucin 1 (MUC-1) on their surface, which suggested they were derived from the tumor. Further, no MUC-1-positive TMPs were detected by light-scatter flow cytometry after breast tumor resection.58
Zwicker and colleagues observed elevated levels of TF-positive MPs in the plasma of patients with pancreatic, breast, colorectal, ovarian, and non-small cell lung cancer, using impedance-based flow cytometry.66 This study included 30 patients with cancer with VTE at study entry and 60 case-matched cancer patient controls without VTE. TF-positive MPs were detected in the plasma of 60% of the patients with cancer who had VTE compared with 27% of patients with cancer without VTE. Again, 50% of the circulating TF-positive MPs in 3 patients with pancreatic cancer expressed MUC-1. Similar to the study by Tesselaar and colleagues,58 plasma levels of TF and MUC-1 double-positive MPs in these 3 patients were reduced after surgical resection of the primary tumor.66
A second study by Tesselaar and colleagues measured plasma MP TF activity levels in 51 unselected patients with cancer who presented with VTE in comparison with case-matched patients with cancer without VTE.91 The cancer types in this study included gastrointestinal tract (n = 27), genitourinary tract (n = 12), and a variety of other cancer types (n = 13). MP TF activity was higher in the patients with VTE compared with the non-VTE patients. Interestingly, patients with pancreatic cancer with VTE had the highest levels of MP TF activity. Increased MP TF activity was found to be associated with decreased survival of patients with cancer. Cancer patients with VTE also had increased plasma TAT levels, and there was a modest correlation between MP TF activity and TAT levels.91 Similar to Tesselaar and colleagues,91 we found increased levels of MP TF activity in patients with cancer who had VTE (n = 53) in comparison with non-VTE patients (n = 13), with the highest levels being in patients with pancreatic cancer.93 A more recent retrospective study also found increased levels of circulating TF-positive MPs identified by flow cytometry in patients with cancer who had VTE compared with patients with cancer without VTE.63
Prospective studies
The earliest prospective study was performed by Khorana and colleagues in 2008.56 MP TF activity was measured in the plasma of 10 patients with pancreatic cancer receiving chemotherapy. Blood samples were drawn before starting chemotherapy and every 4 weeks during a 20-week period. MP TF activity was increased in 2 of the 10 patients, and these 2 patients developed VTE during the study. This is the first study to provide evidence to support the hypothesis that elevated levels of MP TF activity precede thrombosis and may be predictive of VTE in patients with pancreatic cancer.56
Since this initial study, several other studies have evaluated the association between plasma levels of TF-positive MPs and development of VTE in patients with cancer. In the second portion of the study by Zwicker and colleagues, the researchers measured plasma levels of TF-positive MPs in patients with cancer and then followed these patients for the development of VTE.66 This study found a 1-year VTE rate estimate in patients with detectable TF-positive MPs of 34.8% (4 out of 16) compared with 0% (0 out of 44) in patients without detectable TF-positive MPs.66 Another study measured MP TF activity in 122 patients newly diagnosed with multiple myeloma who were eligible for high-dose chemotherapy.94 MP TF activity levels were elevated in patients with multiple myeloma but were not predictive of future VTE events.94 However, the rationale for this study is not clear, as neoplastic plasma cells have not been reported to express TF.95
Van Doormaal and colleagues followed 43 patients with cancer (13 with pancreatic cancer) for a period of 6 months for the development of VTE.64 Five patients developed VTE during the study, 3 of whom were patients with pancreatic cancer. Levels of TF antigen were measured by flow cytometry and enzyme-linked immunosorbent assay, and TF and TF-factor VIIa activity of MPs were measured using the MP TF activity assay and a fibrin generation test with or without an anti-factor VIIa blocking antibody, respectively.64 In addition, levels of PS-positive MPs were measured using flow cytometry and a functional assay. This study found that there was an association between both MP TF activity and MP TF-factor VIIa-dependent fibrin generation and the development of VTE. In contrast, VTE did not correlate with either of the TF antigen-based assays or the level of PS-positive MPs.64 These results indicate that it is important to measure levels of MP TF activity, and not simply levels of PS-positive MPs.96
The most extensive study to date measured MP TF activity in plasma samples from the Vienna cancer and thrombosis study.60 Four different types of patients with cancer (60 pancreatic, 43 gastric, 126 colorectal, and 119 brain) were chosen because these cancers have a relatively high rate of VTE. These patients were followed for up to 2 years for the development of VTE. For many samples, plasma MP TF activity was measured using both the end-point57 and kinetic58 assays. Patients with pancreatic and gastric cancer expressed higher levels of MP TF activity in comparison to patients with brain and colorectal cancer. MP TF activity was associated with decreased survival in patients with pancreatic and gastric cancer. Patients with pancreatic cancer demonstrated borderline significance for an association between MP TF activity and VTE in the end-point assay.60
It is somewhat surprising that the level of circulating MP TF activity was not increased in patients with brain cancer in the above study.60 It is possible that the blood–brain barrier may limit the release of TMPs into the circulation in these patients. Sartori and colleagues found that procoagulant activity of MPs and TF-positive MPs measured by flow cytometry were increased in 61 patients with preoperative glioblastoma multiforme.80,81 Levels of TF-positive MPs were also increased over baseline at 7 days and at 1 month after tumor resection.81 This increase is most likely a result of disruption of the blood–brain barrier during surgery.81 Furthermore, the 11 patients who developed VTE had significantly higher levels of non-tumor-derived TF-positive MPs at baseline than the non-VTE patients.81 However, no increases in MP TF activity were detected in the same samples (R. L. Bradford and N. S. Key, unpublished data).
We have also analyzed MP TF activity in 117 patients with newly diagnosed pancreaticobiliary cancer at all stages. These patients are part of the Roswell Park Cancer Institute Data Bank and Biorepository.97 Blood was collected from each patient after cancer diagnosis. In this study, elevated plasma MP TF activity was associated with the future development of VTE and decreased survival.97
Timing of sample collection for the prediction of future VTE in patients with cancer
A general issue with prospective studies is that the collection of blood samples may occur many months before the thrombotic event. Most studies rely on a single sample to predict future events. We found that in 2 patients with pancreatic cancer, MP TF activity serially increased in the months before the development of thrombosis.56 Further, MP TF activity was found to correlate with VTE in a study with a 6-month follow-up,64 but only weakly in a study with a 2-year follow-up.60 Clearly, it would be better to collect multiple blood samples from each patient, which would allow for the monitoring of levels of TF-positive MPs over time. We have an ongoing study in which we collect 8 blood samples from patients with either advanced pancreatic cancer or advanced colorectal cancer before and during 4 cycles of chemotherapy and monitor for the development of symptomatic and asymptomatic VTE. Using this study design, we will further examine the hypothesis that elevated levels of MP TF activity are predictive of symptomatic and asymptomatic VTE in these patients.
Conclusion
In summary, TF is expressed by tumor cells, and expression is increased in advanced cancer. Tumor cells spontaneously release high levels of TF-positive MPs that are associated with a prothrombotic state and enhanced thrombosis in animal models. In human cancer patient studies, elevated levels of MP TF activity are predictive of VTE in the majority of studies, suggesting that MP TF likely contributes to thrombosis in patients with cancer, particularly in those with pancreatic cancer. However, the current laboratory-based assays need to be improved before they can be used for clinical diagnosis. The clinical utility for the use of plasma MP-TF in the guiding of thromboprophylaxis in patients with cancer also remains to be determined. The first study using plasma MP-TF to guide this type of treatment decision has produced promising results.67 The MicroTEC study found that use of the low–molecular weight heparin enoxaparin for thromboprophylaxis in patients with cancer with high plasma MP-TF reduced VTE and improved patient survival.67
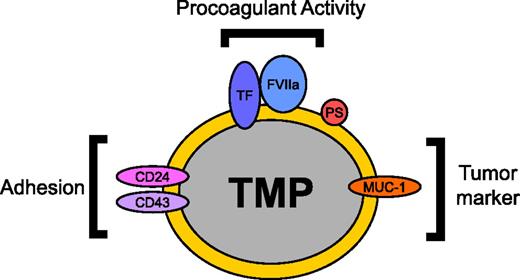
Most studies of patients with cancer focus on measurement of MP TF activity rather than TF activity in other cells. However, levels of MP TF activity may represent the tip of the iceberg in terms of the MP-associated procoagulant activity present in the blood of patients with cancer, as TMPs may be binding to other vascular cell types. Indeed, a recent study analyzed circulating TF activity in the blood of patients undergoing total knee arthroplasty and found that the majority of circulating TF was associated with monocytes, with only ∼5% of the TF activity present in the free MP fraction.98 It is possible that measurement of TF activity in vascular cell populations will be more predictive of thrombosis than MP TF activity. More studies are needed to determine the distribution of TF-positive TMPs in the circulation in animal models and patients with cancer and to characterize the tumor markers and adhesion proteins that are present on the surface of the TMPs (Figure 1). One study found that P-selectin was required for the TMP-induced enhancement of microvascular thrombosis.69 However, it seems likely that other receptors contribute to the delivery of TMPs to thrombosis sites and that these receptors may represent good targets for the development of cancer-specific antithrombotic drugs.
TMP surface proteins and their functions. TMPs are constitutively released from tumors into the circulation. The procoagulant activity of TMPs is mediated by the expression of TF and the exposure of PS on the MP surface. Tumor markers such as MUC-1 can allow for the identification of TMPs in the circulation. Adhesion proteins including P-selectin ligand CD24 and E-selectin ligand CD43 have been proposed to be involved in the binding of TMPs to endothelium and thrombosis sites.2,69 Delivery of TMP TF to the site of thrombosis can then initiate thrombosis. This diagram is an example of proteins that can be expressed on the surface of TMPs. Protein expression on the surface of TMPs varies with each tumor. FVIIa, factor VIIa.
TMP surface proteins and their functions. TMPs are constitutively released from tumors into the circulation. The procoagulant activity of TMPs is mediated by the expression of TF and the exposure of PS on the MP surface. Tumor markers such as MUC-1 can allow for the identification of TMPs in the circulation. Adhesion proteins including P-selectin ligand CD24 and E-selectin ligand CD43 have been proposed to be involved in the binding of TMPs to endothelium and thrombosis sites.2,69 Delivery of TMP TF to the site of thrombosis can then initiate thrombosis. This diagram is an example of proteins that can be expressed on the surface of TMPs. Protein expression on the surface of TMPs varies with each tumor. FVIIa, factor VIIa.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute at the National Institutes of Health, grant numbers R01HL095096-04 and R01HL095096-04S (to N.M. and N. S. Key).
Authorship
Contribution: J.G. and N.M wrote this review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel Mackman, Division of Hematology/Oncology, Department of Medicine, University of North Carolina, Chapel Hill, NC 27599; e-mail: nmackman@med.unc.edu.