In this issue of Blood, Jang et al elegantly demonstrate a previously unidentified role of lymphatics in protecting our body from the gut flora–induced sepsis using a novel mouse model. This study has a significant impact on our current understanding of not only the function of the lymphatics, but also the pathology of sepsis and related intestinal inflammatory diseases.1
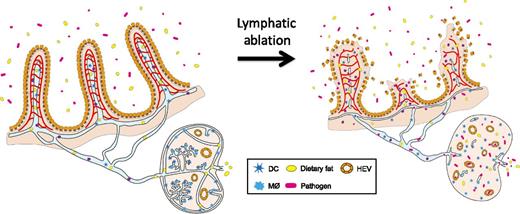
Lymphatics in the intestinal villi and lymph nodes protect us from sepsis. Jang et al1 showed that ablation of intestinal lymphatics and lymph node lymphatics compromises the architecture of the surrounding tissues, including neighboring blood vessels, and leads to infiltration of the intestinal gut flora into the system, causing a systemic infection followed by fatal inflammatory responses, collectively called sepsis. See Figure 7 in the article by Jang et al that begins on page 2151.
Lymphatics in the intestinal villi and lymph nodes protect us from sepsis. Jang et al1 showed that ablation of intestinal lymphatics and lymph node lymphatics compromises the architecture of the surrounding tissues, including neighboring blood vessels, and leads to infiltration of the intestinal gut flora into the system, causing a systemic infection followed by fatal inflammatory responses, collectively called sepsis. See Figure 7 in the article by Jang et al that begins on page 2151.
The lymphatic system has long been thought to function as a secondary fluid drainage system for the blood circulation and has received considerably less scientific and medical attention than the blood vascular system. Many biological and medical textbooks only describe the lymphatics as a network of passive channels that drain and transport tissue fluids, immune cells, and intestinal nutrients with an opportunistic role in tumor metastasis. However, a series of landmark discoveries in recent lymphatic research have significantly changed these old-fashioned views of the lymphatics. For example, dermal lymphatic vessels in the skin have been recently found to control salt-sensitive interstitial fluid volume and blood pressure.2 In addition, recent studies have shown that lymphatic vessels are essential for reverse cholesterol transport.3,4
In this issue of Blood, another discovery in the neglected field of lymphatic research is reported by Jang et al.1 They initially intended to determine the effect of systemic lymphatic ablation on tissue fluid homeostasis using a novel mouse model that selectively expresses the diphtheria toxin receptor in lymphatic endothelial cells. Unexpectedly, however, the mice died within a few days on administration of diphtheria toxin, before the appearance of signs of systemic fluid accumulation. Detailed follow-up studies have causally associated the lethality with typical symptoms of sepsis, including increased serum endotoxin levels and decreased lymphocyte and platelet numbers as well as enlarged Peyer patches that are densely packed with immune cells. The authors then found that, although the majority of lymphatic vessels appeared to be uncompromised, lacteals (specialized lymphatic capillaries that absorb dietary lipids in the small intestine and lymph node lymphatics) immediately disappeared within several hours of toxin administration. Interestingly, this acute ablation of lacteals was found to cause disintegration of adjacent blood capillaries and subsequently lead to dissolution of the entire architecture of the villi, triggering severe acute intestinal inflammation and sepsis (see figure).
This study provides us several lines of important information. Among them, the protective role of the intestinal tissue integrity will be newly added to the expanding list of lymphatic functions. This point is particularly exciting considering that more experimental data point dysfunctional lymphatics as a key contributing factor to the pathogenesis of Crohn disease, a type of inflammatory bowel disease that is often associated with compromised intestinal villi.5
In addition, the animal model used in the current study could potentially be developed as a new tool to study sepsis. Sepsis is causally defined as a systemic infection by bacteria, viruses, fungi, and/or parasites mainly in blood and is clinically manifested as systemic inflammatory response syndrome in the presence of infection, including low blood pressure; cognitive impairment; metabolic acidosis; elevated heart rate; respiratory dysfunction; capillary leakiness; decreased lymphocyte counts; hypothermia or hyperthermia; and, eventually, multiple organ dysfunction.6 This overwhelming, dysregulated systemic immune response claims millions of lives worldwide each year. Numerous surgical and nonsurgical animal models have been developed to date, including cecal ligation and puncture and lipopolysaccharide-based toxemia models. Because sepsis can occur for multiple reasons and can be aggravated by various risk factors, not a single animal model is perfect enough to recapitulate most of the clinical symptoms of sepsis. Notably, although decades and billions of dollars have been spent on these animal models, some recent studies alarm us about the uncomfortable possibility that these rodent models rather poorly mimic human inflammatory disease and thus may have seriously misled our fights against this deadly disease. This is especially true when judged by huge differences in the genomic profiles between mouse models and patients with sepsis.7,8 This possibility haunts the troubling fact that none of nearly 150 drug candidates for sepsis tested during past decades has landed to the clinics.
Yes, mice are not humans, and yes, they are “experimental” models. Despite the substantial gap between 2 species, the current animal models have helped us tremendously to understand the disease. Moreover, considering the complex and heterogenic nature of sepsis, it would be better to have multiple animal models that could recapitulate different aspects of sepsis. In this context, the study by Jang et al provides us another useful model against this very challenging disease. Although the authors did not fully address many important features of sepsis from their mice, the findings of elevated endotoxin levels, lower lymphocyte counts, and dissolution of villous capillaries indeed warrant further studies of their mouse model. Sepsis-like phenotypes that are caused by compromised intestinal and lymph node lymphatics are quite noteworthy and possibly present some features that other mouse sepsis models have not clearly demonstrated.
Conflict-of-interest disclosure: The author declares no competing financial interests.