Abstract
Therapeutic options for the management of venous thromboembolism (VTE) in patients with cancer remain very limited. Although low-molecular-weight heparin monotherapy has been identified as a simple and efficacious regimen compared with an initial parenteral anticoagulant followed by long-term therapy with a vitamin K antagonist, many clinical questions remain unanswered. These include optimal duration of anticoagulant therapy, treatment of recurrent VTE, and the treatment of patients with concurrent bleeding or those with a high risk of bleeding. Treatment recommendations from consensus clinical guidelines are largely based on retrospective reports or extrapolated data from the noncancer population with VTE, as randomized controlled trials focused on cancer-associated thrombosis are sorely lacking. Furthermore, with improvements in imaging technology and extended survival duration of patients with cancer, we are encountering more unique challenges, such as the management of incidental VTE. Clinicians should be aware of the limitations of the novel oral anticoagulants and avoid the use of these agents because of the paucity of evidence in the treatment of cancer-associated thrombosis.
Introduction
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in patients with cancer.1 Although deep vein thrombosis (DVT) and pulmonary embolism (PE) are the most commonly encountered venous thrombotic complications, other vascular territories, such as the splanchnic veins and upper extremity venous system, can also be involved. With the increasing age and cancer prevalence of our population, enhanced detection of incidental thrombosis and greater thrombogenicity of multiagent chemotherapeutic regimens, we have observed a steady increase in the incidence of cancer-associated thrombosis during the past 2 decades.2,3 A leading cause of death in patients with cancer,4 thrombosis is associated with higher mortality risk, irrespective of cancer stage.5,6
Unfortunately, despite the burden of VTE in oncology patients, there has been limited advancement in the management of cancer-associated thrombosis since the introduction of low-molecular-weight heparin (LMWH) for long-term therapy. Anticoagulant therapy in malignancy remains burdensome and is viewed as having a negative impact on patient quality of life. It is also associated with a high risk of bleeding and may limit the therapeutic options to treat the underlying cancer.7
In this review, we outline the current evidence and consensus guideline recommendations for the initial and long-term management of the first and recurrent episodes of VTE in patients with cancer. We also discuss the management of commonly encountered thrombotic complications, recognizing the lack of rigorous evidence in these areas, such as incidentally detected VTE, splanchnic vein thrombosis, catheter-related thrombosis, and VTE treatment in patients with a high risk of bleeding. We close with a critical look at the use of the novel oral anticoagulants (NOACs) in the treatment of cancer-associated thrombosis.
Initial management of a first episode of cancer-associated VTE
Choice of anticoagulant
Options for the initial treatment of cancer-associated thrombosis include LMWH, unfractionated heparin (UFH), and fondaparinux. Although studies directly comparing these agents are lacking in oncology patients, data extrapolated from subgroup analysis of trials in unselected patients showed no difference in efficacy between LMWH and UFH in patients with cancer.8 However, a statistically significant reduction in mortality risk with LMWH at 3 months of follow-up has been noted. The reason for this survival benefit is unknown, but research exploring the antineoplastic properties of LMWH is ongoing. In addition to better efficacy, LMWH provides other advantages vs UFH, including lower cost (because hospitalization and laboratory monitoring are not required) and simple dosing (because the total daily dose is based on body weight). LMWH is also associated with a lower risk for heparin-induced thrombocytopenia (HIT).9
Data on the use of fondaparinux for the initial management of DVT and PE in patients with cancer are also derived from post hoc subgroup analysis. In the Matisse trials, the 3-month rate for symptomatic, recurrent VTE was higher for fondaparinux vs enoxaparin in DVT treatment (12.7% vs 5.4%) but was lower for fondaparinux vs UFH in PE treatment (8.9% vs 17.2%).10 Like LMWH, fondaparinux is administered as a once-daily, weight-based subcutaneous injection. Fondaparinux is rarely associated with the development of drug-induced thrombocytopenia and has been used off label for the management of HIT.11 Barriers to its use in oncology patients include a relatively long half-life of 17 to 21 hours, the lack of a reversal agent, and 100% dependence on renal clearance.12
On the basis of currently available evidence, LMWH is the recommended anticoagulant for the initial therapy of VTE in most patients with cancer (Table 1).13-16 However, UFH can be used in those with severe renal impairment (creatinine clearance [CrCl] <30 mL/min) given its shorter half-life, reversibility with protamine sulfate, and dependence on hepatic clearance. Fondaparinux is a reasonable choice in patients with a history of HIT.
Use of thrombolysis in cancer-associated VTE
As most trials of thrombolytic therapy exclude patients with cancer because of a perceived higher risk of bleeding, evidence for thrombolysis in patients with malignancy is limited to small single-center, retrospective series. These studies compared the degree of clot lysis and short-term complications between patients with vs those without cancer who had presented with extensive DVT or PE.17-19 Although comparable results between cancer and noncancer patient groups were observed, these studies provide a low level of evidence because of insufficient statistical power and patient selection bias. Nonetheless, there is no strong evidence to routinely exclude patients for consideration of thrombolysis on the basis of the presence of malignancy alone. Because the safety, cost-effectiveness, and long-term benefit of thrombolysis remain uncertain,20 it is prudent to review each patient carefully and exclude patients with central nervous system lesions or other risk factors for bleeding.
Long-term management of a first episode of cancer-associated VTE
Choice of anticoagulant
Although vitamin K antagonists (VKAs) have been the mainstay agents for long-term management and secondary prophylaxis of acute VTE in patients without cancer,21 their use is problematic in oncology patients. VKAs are less effective in patients with cancer, with rates of recurrent VTE threefold higher than in patients without cancer despite maintenance of the international normalized ratio (INR) within the therapeutic range.22,23
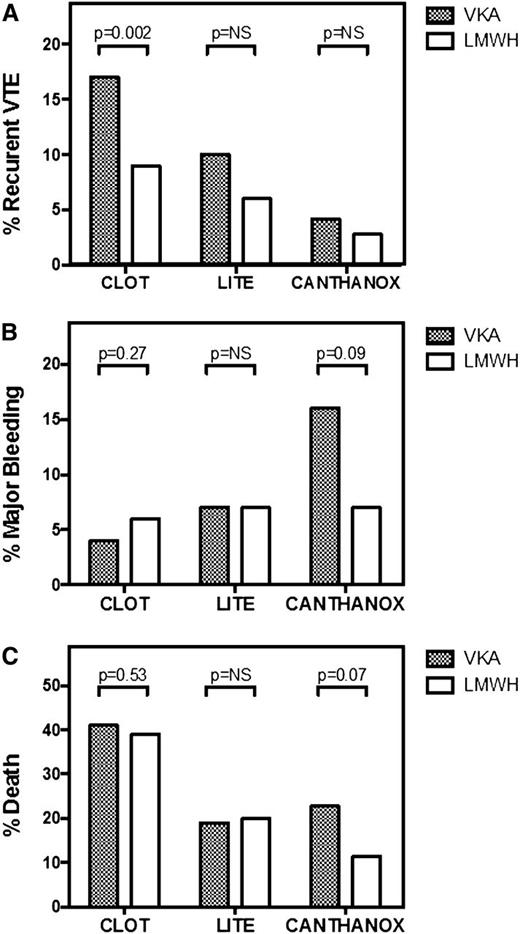
Several open-label, randomized controlled trials have compared LMWH with VKA therapy for long-term management of cancer-associated PE or proximal DVT (Figure 1).24-27 Three different LMWH preparations, enoxaparin, tinzaparin, and dalteparin, were investigated for 3 to 6 months in similarly designed studies. Overall, the results from these trials provide consistent evidence of improved efficacy of LMWH vs VKA in the prevention of recurrent VTE in patients with cancer-associated VTE. A meta-analysis of 7 studies confirms this finding, reporting a relative risk reduction of 53%.28
Comparison of randomized controlled trials of different preparations of LMWH vs VKA for the long-term management of cancer-associated thrombosis. Recurrent VTE (A), major bleeding episodes (B), and mortality (C) during the anticoagulant treatment period are shown for the CLOT24 (6 months of dalteparin or VKA), LITE25 (3 months of tinzaparin or warfarin), and CANTHANOX27 (3 months of enoxaparin or warfarin) trials. NS, not statistically significant.
Comparison of randomized controlled trials of different preparations of LMWH vs VKA for the long-term management of cancer-associated thrombosis. Recurrent VTE (A), major bleeding episodes (B), and mortality (C) during the anticoagulant treatment period are shown for the CLOT24 (6 months of dalteparin or VKA), LITE25 (3 months of tinzaparin or warfarin), and CANTHANOX27 (3 months of enoxaparin or warfarin) trials. NS, not statistically significant.
In addition to improved efficacy vs VKA therapy, LMWH also offers other advantages including (a) no need for laboratory monitoring of its anticoagulant activity; (b) a shorter half-life that facilitates temporary interruption for procedures or thrombocytopenia; (c) limited drug interactions; and (d) no food interactions or reliance on oral intake or gastrointestinal tract absorption. As a result, LMWH is recommended for both initial and long-term anticoagulation in cancer-associated thrombosis by major consensus guidelines (Table 1).13-15,21 The high cost associated with LMWH therapy and the requirement for daily subcutaneous injections are the major barriers to its use. Yet, qualitative studies have reported that patient acceptance of daily injections is quite favorable.29 In fact, in those who had used warfarin and LMWH therapy, most prefer the convenience and “empowerment” with LMWH. If LMWH is unavailable, the American Society of Clinical Oncology (ASCO) 2013 VTE Prevention and Treatment Guideline recommends the use of VKA with a target INR of 2 to 3 as an acceptable alternative.14
Anticoagulation in patients with renal impairment
Renal impairment is not uncommon among patients with malignancy. Because LMWH is partially cleared by renal excretion and metabolism, drug accumulation is expected with long-term use in those with significant renal insufficiency (CrCl <30 mL/min). Of the major LMWH preparations, tinzaparin appears to have the lowest potential to accumulate, whereas enoxaparin shows significant accumulation in renal insufficiency.30 This property reflects the chain length of the LMWH, with the longer-chain tinzaparin being partially cleared by the reticuloendothelial system.30,31
Limited data are available on the use of LMWH in patients with significant renal dysfunction, but they do indicate that the risk of bleeding is higher in patients with renal impairment.32,33 Manufacturer-recommended dose reduction in renal impairment exists for enoxaparin but not for other LMWH preparations.34 Most experts and guidelines recommend dose adjustment based on anti-factor Xa activity in patients with a CrCl <30 mL/min.13,21 If anti-factor Xa monitoring is not readily available, VKA therapy is likely a safer option for long-term anticoagulation in these patients. A warning against the use of tinzaparin in elderly patients with renal impairment has been issued by the US Food and Drug Administration after the results of an interim analysis of a randomized trial comparing tinzaparin with UFH for initial VTE therapy in this vulnerable population.35 Only 33 patients with a history of ongoing malignancy were enrolled in this study; 20 of them were in the tinzaparin group.36
Duration of anticoagulation and anticoagulant options for extended therapy
The decision regarding the continuation of anticoagulation beyond the first 3 to 6 months is largely based on weighing the risk for recurrent thrombosis against the risk of major bleeding. This evaluative process is best developed in patients with unprovoked VTE, in whom studies have been done to determine whether biomarkers, radiologic imaging, and clinical prediction models can identify patients with a sufficiently high risk for recurrent thrombosis to benefit from extended anticoagulation, or an acceptably low risk to allow discontinuation of anticoagulation.37-39 A clinical model to predict the risk for recurrent VTE during anticoagulation therapy in cancer-associated thrombosis has been proposed but not yet validated.40 In addition, studies regarding the optimal duration of anticoagulant therapy are lacking in oncology patients.
Given that the risk for recurrent thrombosis in patients with active cancer is high even while they are receiving anticoagulation, it is generally recommended that extended anticoagulation be considered in this population.14,21 It has not been established whether risk factors for a first episode of VTE, such as metastatic or progressive disease and ongoing chemotherapy, will also increase the risk for recurrent VTE, but it has been generally accepted that continuing anticoagulation in patients with these ongoing risk factors is warranted.13-15 Patients given extended anticoagulation require frequent reassessment to review the risk-benefit balance of continuing anticoagulant therapy. Factors that need to be taken into account, aside from the risk for recurrent VTE and the risk of bleeding, include the status of the malignancy, type of cancer treatment (if any), quality of life, and patient preference.
The choice of anticoagulant for extended anticoagulant therapy (beyond 6 months) also has not been investigated. A randomized trial is currently addressing this question.41 Patient preference and tolerance of previous therapy play significant roles in this decision. Thus far, long-term toxicity associated with VKA or LMWH has not been a concern, although it remains controversial whether prolonged exposure to LMWH accelerates clinically significant bone loss.42 As with duration of therapy, the anticoagulant of choice needs to be discussed with each patient and individualized.
Inferior vena cava (IVC) filters
Patients with cancer are frequent recipients of IVC filters. Yet, data on the efficacy and safety in this population are limited to retrospective single-center series or anecdotal reports.43,44 Rates of recurrent VTE up to 32% have been reported in patients with cancer treated with IVC filters, and fatal PE after filter insertion has been well documented.45
Evidence on the safety and efficacy of IVC filters in the general population is also sparse. Only 1 randomized trial has been done to evaluate filter placement in patients with proximal DVT who are at high risk for PE. In the PREPIC (Prévention du Risque d'Embolie Pulmonaire par Interruption Cave) trial, the use of permanent IVC filters in conjunction with anticoagulation resulted in a decreased incidence of PE at the expense of an increase risk for recurrent DVT and without any reduction in overall mortality rate during 8 years of follow-up.46,47 Results for the included 56 patients with cancer have not been published. Most importantly, the value of an IVC filter in patients who cannot receive anticoagulation, the major indication for filter insertion, has not been examined in prospective studies.
Complications associated with IVC filters raise further concern about the appropriateness of their use. Insertion problems occur in 4% to 11% of patients, and long-term adverse effects such as thrombosis of the IVC or lower extremity veins occur in 4% to 32%.48 A high incidence of mechanical or device failures associated with retrievable filter models led the US Food and Drug Administration to issue a Safety Alert in 2010.49 These problems include filter migration, strut fracture with embolization, and caval perforation.50,51
Given the high rates of complications and the absence of data to support their efficacy, IVC filters should be restricted to patients with acute VTE and contraindications to anticoagulation. Their use in patients with recurrent thrombotic events despite standard anticoagulant therapy goes against biological rationale because filters do not treat the underlying thrombotic condition, and the presence of an intravascular foreign body is likely to promote thrombus formation, which can occur proximally or distally to the filter. In addition, the use of IVC filters may provide a sense of false security regarding the risk for recurrent PE, causing delays or discontinuation of anticoagulant therapy. If retrievable filters are placed, efforts should be made to remove the device and reinitiate anticoagulation as soon as the high-risk period for bleeding has passed.
Treatment of recurrent VTE during anticoagulant therapy
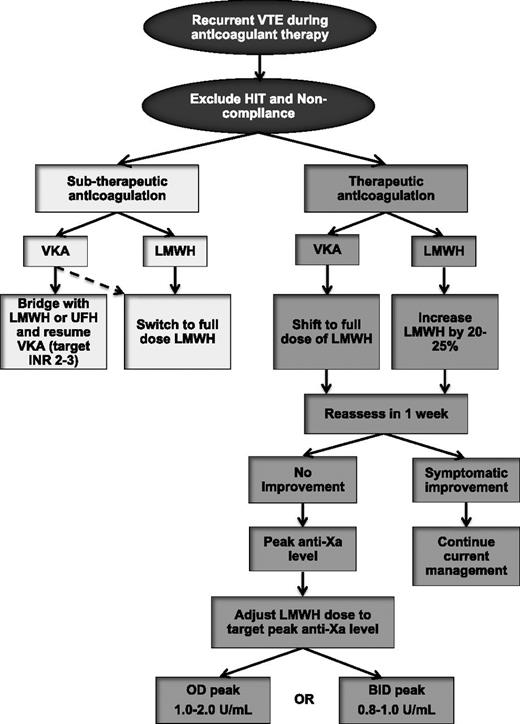
Failure of anticoagulation in cancer-associated thrombosis is common, but there is a paucity of data to help guide treatment. An empiric approach for the treatment of recurrent VTE during anticoagulant therapy is outlined in Figure 2. Once recurrent VTE is confirmed, it is essential that HIT be excluded in patients who were first exposed to LMWH or UFH within the past 10 to 14 days. Compliance should also be reviewed. In patients who experience a recurrent event while receiving VKA therapy and have a subtherapeutic INR, options include continuation of VKA after a bridging period with LMWH (or UFH) or switching to LMWH monotherapy. The latter is likely a better option, especially in patients who had unstable INR values and the time-in-therapeutic range was low. For patients who experienced warfarin failure while the INR values had been therapeutic, transition to LMWH is recommended given its greater efficacy vs warfarin.24,25,27,52 Recurrent VTE events during LMWH therapy can be treated with a dose escalation of LMWH. A retrospective study of 70 patients with cancer with recurrent VTE demonstrated that transition to LMWH (in patients receiving VKA therapy at the time of recurrence) or LMWH dose escalation by 20% to 25% (in patients receiving LMWH at recurrence) prevented additional VTE in 91% of patients during a minimum of 3 months of follow-up.53 Only 1 patient had a major bleeding event. If another recurrent VTE episode occurs after the first dose escalation, further dose increase or twice-daily dosing of LMWH are reasonable options. The use of anti-factor Xa levels may help to further tailor LMWH escalation (Figure 2), although published evidence to support this strategy is lacking.
Management algorithm of recurrent VTE in patients with cancer. BID, twice-daily dosing; INR, international normalized ratio; OD, once-daily dosing.
Management algorithm of recurrent VTE in patients with cancer. BID, twice-daily dosing; INR, international normalized ratio; OD, once-daily dosing.
Treatment of incidental VTE
Incidental or unsuspected VTE is defined as evidence of thrombosis detected on imaging studies performed for other indications such as cancer staging.54 Retrospective studies in unselected oncology patients have demonstrated incidental VTE rates of up to 6%,55,56 with thrombotic events evenly distributed between PE and/or DVT and intra-abdominal thrombosis.55 A prevalence of incidental PE of 3.1% was reported in a meta-analysis that included data from more than 6000 oncology patients.57 Incidental VTE also represents a significant proportion of thrombotic complications in patients with cancer, comprising up to 60% in large series.58
Studies have suggested that, like suspected or symptomatic VTE, the occurrence of incidental VTE can have a negative impact on both patient quality of life and clinical outcomes. Patients with incidental PE often have symptoms such as fatigue and shortness of breath.59 In a retrospective case-control study comparing 51 unselected oncology patients with incidental PE and 144 patients with symptomatic PE, the 12-month rates of recurrent VTE, bleeding, and mortality were similar between the groups.60 A retrospective cohort of 135 consecutive patients with pancreatic cancer reported similar findings.61 In another study, a comparison of cancer patients with and without incidental PE matched for age, cancer diagnosis, and stage of disease at the time of a restaging computed tomography scan of the chest found that patients with incidental PE had a higher mortality rate than those without PE.62
Whether anticoagulation is indicated or beneficial in patients with incidental VTE remains controversial. Evidence is limited to 2 retrospective studies in patients with lung and pancreatic cancer. In a cohort of 113 patients with lung cancer and incidental PE, the 62 patients who did not receive anticoagulation had higher mortality rates compared with patients who were treated.63 Similarly, in patients with pancreatic cancer with incidental VTE, the use of anticoagulant therapy was associated with a 70% reduction in mortality rate.61 However, these studies are potentially biased to favor anticoagulation because anticoagulant therapy is likely to be withheld in those with a short life expectancy.
Nonetheless, based on published literature to date, it is recommended that patients with incidental DVT and PE receive therapeutic anticoagulation if there are no contraindications.14,21 However, caution should be exercised in cases where the diagnosis of PE or DVT is questionable, especially for isolated subsegmental PE. Confirming the diagnosis with the appropriate testing (ie, computed tomographic pulmonary angiography or compression venous ultrasonography) is strongly encouraged in such cases.
Treatment of cancer-associated thrombosis in patients with a high risk of bleeding
Bleeding is frequently associated with anticoagulant use in patients with cancer. In a prospective study including 181 oncology patients receiving VKA for the treatment of DVT, the 1-year cumulative incidence of major bleeding was 12.4%, with one third of the bleeding events occurring during the initial phase of anticoagulation.23 Although subtherapeutic INR values are associated with recurrent VTE, there appears to be no correlation between the INR level and bleeding in patients with cancer.22,64 Bleeding complications are not restricted to VKA therapy, as prospective randomized controlled trials comparing LMWH and VKA therapy for cancer-associated VTE have reported similar bleeding rates.24-26 Features specific to oncology patients that contribute to bleeding include the extent, location, and histologic features of the cancer, need for invasive diagnostic or treatment procedures, and the development of thrombocytopenia from chemotherapy or from the underlying malignancy. Other comorbidities such as renal impairment and coagulopathy from liver dysfunction, disseminated intravascular coagulopathy, or sepsis further predispose them to bleeding.
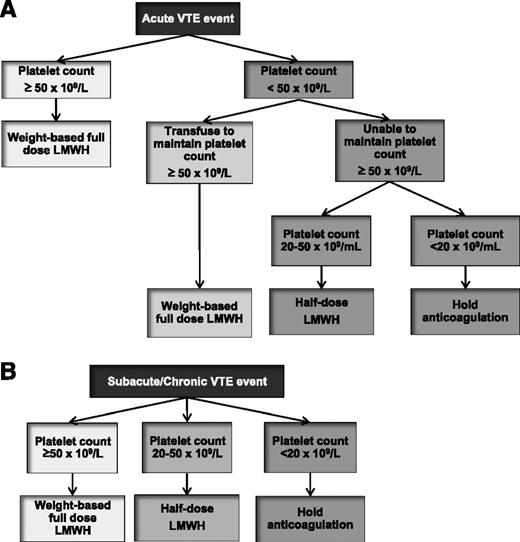
Because of potentially serious and life-threatening bleeding complications, all patients require an individualized assessment of their bleeding risk before the initiation of anticoagulation. Current and potential bleeding sources should be identified and managed, and the risk of serious bleeding should be weighed against the severity of the thrombotic event and risk for recurrent VTE. In patients with minor bleeding, anticoagulation may be continued as long as close follow-up is available. In patients with absolute contraindications to anticoagulation, the risk of bleeding likely outweighs the benefit of treatment, and anticoagulants should be withheld.14 In these patients, follow-up imaging should be performed to assess for thrombus progression, and IVC filter insertion can be considered. If severe cancer- or chemotherapy-induced thrombocytopenia is present, platelet transfusions may be used to allow anticoagulation. Most experts agree that therapeutic anticoagulation with LMWH may be administered if the platelet count can be maintained above 50 × 109/L.65 For platelet counts between 20 and 50 × 109/L, half-dose LMWH can be administered with close follow-up for possible bleeding. If platelet count is <20 × 109/L, therapeutic doses of anticoagulation should be held. Limited evidence from case series suggests that the use of prophylactic doses of LMWH can be tolerated in patients with platelet counts <20 × 109/L with associated resolution of thrombotic symptoms.66 VKA therapy should be avoided in patients with severe thrombocytopenia from the prolonged anticoagulant effect and unpredictable dose response. Figure 3 illustrates a reasonable approach to the treatment of cancer-associated thrombosis in patients with thrombocytopenia.65
Management algorithm of VTE in patients with cancer and thrombocytopenia. Management of acute VTE (<1 month) and subacute or chronic VTE (≥1 month) are outlined in panels A and B, respectively.
Management algorithm of VTE in patients with cancer and thrombocytopenia. Management of acute VTE (<1 month) and subacute or chronic VTE (≥1 month) are outlined in panels A and B, respectively.
Management of VTE in patients with intracranial malignancies is particularly challenging because of the fear of intracranial hemorrhage. No randomized controlled data exist for management of patients with primary or metastatic intracranial tumors and VTE; however, small retrospective studies indicate that anticoagulation can be safely used. Anticoagulation with intravenous UFH followed by VKA or subcutaneous UFH has been associated with rates of symptomatic intracranial hemorrhage between 0% and 7%.67-70 Similar results have been reported with LMWH in patients with glioma or metastatic brain tumors with a trend toward improved survival duration.71-73 The ASCO 2013 VTE Guideline recommends treating patients with intracranial malignancies with standard anticoagulation.14
Treatment of catheter-related thrombosis
Treatment of catheter-related thrombosis is largely inferred from treatment of DVT because randomized controlled trials evaluating management strategies have not been performed. Whether line removal is necessary (to eliminate the thrombotic source), harmful (because it can trigger PE), or beneficial (in shortening or avoiding anticoagulation therapy) has not been studied in clinical trials. A small prospective study evaluating the use of anticoagulation alone reported that in 74 patients with active cancer, LMWH for 5 to 7 days followed by warfarin with a target INR of 2.0 to 3.0 for 3 months resulted in no recurrent VTE at the expense of 3 episodes of major bleeding, including 1 fatal hemorrhage.74 After 3 months of treatment, 57% of patients had functional catheters in situ, whereas the remainder had catheter removal for reasons other than progressive thrombosis or device failure. A small patient series observed that a short course of low-dose LMWH is effective and safe in treating catheter-related thrombosis in patients with severe thrombocytopenia.75
To date, published data and clinical experience suggest that catheter-related thrombosis is associated with a low risk for thrombosis recurrence and postthrombotic syndrome.76,77 Therefore, conservative treatment is recommended. A sensible approach is to remove the catheter only if (1) central venous access is no longer required; (2) the device is nonfunctional or defective; or (3) line-related sepsis is suspected or documented. Unless contraindicated, therapeutic anticoagulation should be given using either LMWH alone or LMWH followed by warfarin therapy. A short period of anticoagulation (3-5 days of LMWH) may even salvage some thrombosed catheters and obviate the need to remove and replace the line. Anticoagulation is recommended for a minimum of 3 months and while the catheter remains in place.
Treatment of splanchnic vein thrombosis in patients with cancer
Splanchnic vein thromboses, involving the portal, splenic, mesenteric, or hepatic veins, are uncommon in the general population, but significant rates have been reported in patients with intraabdominal malignancies.78 In a retrospective single-institution study of 135 consecutive patients with pancreatic adenocarcinoma, incidental splanchnic vein thrombosis was present in 23%.60
Management of splanchnic vein thrombosis has not been well studied. Limited experience is available from studies that included both patients with and without cancer. In a retrospective cohort of 832 patients with splanchnic vein thrombosis, of which 27% had underlying cancer, warfarin therapy and gastrointestinal tract varices were independent predictors of bleeding.79 Furthermore, VTE recurrence was not reduced after anticoagulant therapy. An international registry of 613 patients with splanchnic vein thrombosis reported that most patients are treated with anticoagulation and that the risk of major bleeding is low.80 Whether such results apply to cancer-related splanchnic vein thrombosis is not known.
In patients with acute, symptomatic splanchnic vein thrombosis without contraindications to anticoagulation, guidelines recommend the use of anticoagulant therapy.21 For patients with incidentally detected splanchnic vein thrombosis, there is no specific guidance on treatment. It is reasonable to withhold anticoagulation if the patient is truly asymptomatic, especially if radiologic evidence indicates that the thrombus is chronic in nature. Repeated imaging is prudent to detect thrombus progression if anticoagulation is not given.
Use of NOACs in cancer-associated thrombosis
The development of NOACs that directly inhibit factor Xa or thrombin is a milestone achievement in the prevention and treatment of VTE. These agents are more attractive to patients and clinicians because they are taken by mouth in fixed doses once or twice daily, have few drug and food interactions, and do not require laboratory monitoring. Dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, 2 direct factor Xa inhibitors, are the forerunners in this class of agents. These drugs have been shown to be effective in VTE prophylaxis after major hip and knee arthroplasty and in stroke prevention in patients with nonvalvular atrial fibrillation.81 They are also noninferior to warfarin for the prevention of recurrent VTE without an increased risk of bleeding, and rivaroxaban has received regulatory approval as monotherapy in the treatment of DVT.82-87 All 3 agents are effective and safe for long-term secondary VTE prophylaxis in patients who have already received 6 to 12 months of anticoagulation.82,85,87 Unfortunately, very small numbers of patients with cancer were included in these trials, and the results of this patient subgroup have not been published. A small phase 2 study evaluating the safety and tolerability of apixaban found a low risk of major bleeding (2.2%) during 12 weeks of therapy in 125 patients with metastatic or advanced cancer without thrombosis.88 No studies have specifically addressed the treatment of cancer-associated VTE using these direct inhibitors.
Despite the undeniable practical advantages of these agents vs VKA and LMWH therapy for the prevention and treatment of cancer-associated thrombosis, important and clinically relevant concerns prevail regarding the extrapolation of published results to the cancer population. These include the small number of highly selected patients with cancer (approximately 5%) enrolled in each study and the use of warfarin or placebo rather than LMWH in the control group. In addition, although these anticoagulants have fewer drug interactions than VKAs, interactions do exist with some chemotherapeutic agents (Table 2). Whether these interactions are clinically significant is not known. Finally, gastrointestinal tract problems in patients with cancer can potentially alter drug delivery and absorption, and higher rates of gastrointestinal tract bleeding have been reported with dabigatran compared with warfarin.81 These shortcomings are compounded by the lack of reversal agents to rapidly normalize hemostasis and the lack of widely available laboratory assays to measure the anticoagulant activity. Clinicians need to discuss these limitations to fully inform their patients with cancer and know that the current ASCO Guideline does not recommend the use of these new agents.14 Clinical trials are strongly encouraged to address this and the many other unmet clinical needs in patients with cancer-associated thrombosis.
Authorship
Contribution: A.Y.Y.L. and E.A.P. wrote the manuscript.
Conflict-of-interest disclosure: A.Y.Y.L. reports receiving research funding from LEO Pharma and honoraria from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, LEO Pharma, Pfizer, and Sanofi Aventis. The remaining author declares no competing financial interests.
Correspondence: Agnes Y. Y. Lee, University of British Columbia and Vancouver Coastal Health Diamond Health Care Centre, 2775 Laurel St, 10th Floor, Vancouver, BC, Canada; email: alee14@bccancer.bc.ca.