Abstract
In this review, we examine the evidence that neutrophil extracellular traps (NETs) play a critical role in innate immunity. We summarize how NETs are formed in response to various stimuli and provide evidence that NETosis is not universally a cell death pathway. Here we describe at least 2 different mechanisms by which NETs are formed, including a suicide lytic NETosis and a live cell or vital NETosis. We also evaluate the evidence for NETs in catching and killing pathogens. Finally, we examine how infections are related to the development of autoimmune and vasculitic diseases through unintended but detrimental bystander damage resulting from NET release.
Introduction
The polymorphonuclear leukocyte (PMN), or neutrophil, has long been recognized as the infantry of the innate immune system, rapidly deploying to sights of injury and infection. Considerable knowledge has accumulated demonstrating how these cells contribute to inflammation and host defense. In particular, the mechanisms of neutrophil recruitment, phagocytosis, nicotinamide adenine dinucleotide phosphate (NADPH) oxidative burst, and toxic granule-dependent microbial killing have been elucidated in great detail. However, this conventional paradigm dramatically shifted with the observation that stimulated PMNs could release extracellular nucleic acids decorated with histones and granular proteins capable of entrapping exogenous bacteria. The discovery of these neutrophil extracellular traps (NETs) has spawned an entirely new field of granulocyte investigation. Since the initial description of NETs, as with most discoveries, more questions have been generated than answers. In this review, we will focus on the controversial aspects of NET release and the gaps in our knowledge and delve into the role of NETs as true mediators of host defense. The role of NETs during thrombosis, as well as their contribution to bystander tissue injury and autoimmune syndromes, will also be discussed.
The “osis” of NETosis
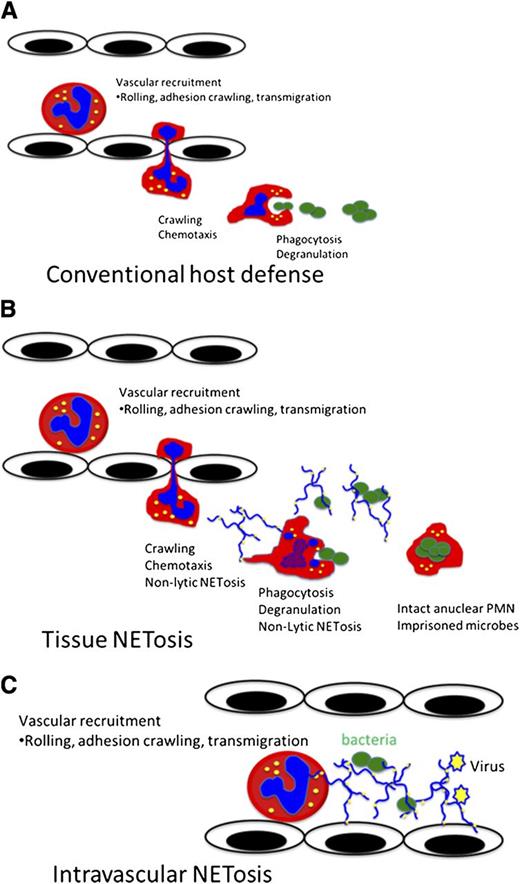
It remains controversial whether NET release represents an active and specific physiological host defense mechanism or is simply a consequence of cellular rupture due to toxins or trauma. Importantly, critics of the concept of active NET release, also known as NETosis, have questioned how this form of cell death relates to currently known death mechanisms including necrosis, apoptosis, phagocytosis-induced cell death, pyroptosis, phagoptosis, and cell lysis. Skeptics remain unconvinced that the release of PMN NETs could have gone unnoticed by pathologists and scientists for so long. However, a careful review of the literature does suggest that NETosis has been observed over the years.1-3 Additionally, critics point out that a death pathway, such as NETosis, would certainly negate the conventional PMN host defensive functions, such as chemotaxis, phagocytosis, and pathogen killing and elimination (Figure 1). Here we will describe the relationship of classic suicidal NETosis to currently understood forms of cell death and introduce the concept of a non–cell death, or as we call it, vital NETosis.
Vital NETosis allows PMNs to maintain conventional host defensive functions. (A) Conventional neutrophil host response incudes the recruitment cascade, emigration, chemotaxis, phagocytosis, and microbial killing. (B) Vital NETosis aids in containing local infections, such as gram-positive cellulitis, by allowing PMNs to rapidly release NETs and continue to chemotax and phagocytose live bacteria. Additionally, the live NET-releasing PMNs are able to maintain their membrane integrity, thereby imprisoning the captured bacteria. (C) Intravascular NET release optimizes the capture of both bacteria and viruses within the blood stream. Intravascular NETosis may also contribute to immunothrombosis.
Vital NETosis allows PMNs to maintain conventional host defensive functions. (A) Conventional neutrophil host response incudes the recruitment cascade, emigration, chemotaxis, phagocytosis, and microbial killing. (B) Vital NETosis aids in containing local infections, such as gram-positive cellulitis, by allowing PMNs to rapidly release NETs and continue to chemotax and phagocytose live bacteria. Additionally, the live NET-releasing PMNs are able to maintain their membrane integrity, thereby imprisoning the captured bacteria. (C) Intravascular NET release optimizes the capture of both bacteria and viruses within the blood stream. Intravascular NETosis may also contribute to immunothrombosis.
Suicidal NETosis
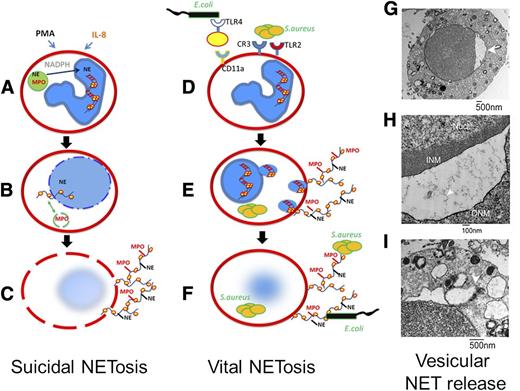
In 1996, neutrophil suicide, distinct from either necrosis or apoptosis, was described following chemical stimulation with phorbol 12-myristate 13-acetate (PMA).3 This form of suicide followed a stepwise progression of chromatin decondensation, nuclear swelling, spilling of the nucleoplasm into the cytoplasm, and finally membrane perforation. The potential importance of this observation went unrecognized until the Zychlinsky laboratory reported that PMA-induced suicide resulted in the release of a novel host defense structure, named NET.4 Using an in vitro system, this group reported that PMA, or interleukin-8 (IL-8), elicited the release of weblike structures of DNA coated with histones and elastase. Using detailed in vitro cellular imaging, Fuchs et al5 defined NET release as an NADPH oxidase–dependent cellular death process requiring chromatin decondensation, followed by nuclear envelope disintegration and mixing of nucleic acids and granule proteins within a large intracellular vacuole (Figure 2). Finally, after intracellular assembly, NETs were released via plasma membrane perforation and cell lysis. This process occurred hours following the inciting stimuli, and once released, these DNA structures bound both gram-negative and gram-positive bacteria. These studies implicated PMA-induced DNA release as a novel host defense form of beneficial suicide, subsequently coined “NETosis.”6
Suicidal NETosis vs vital NETosis. (A-C) Suicidal NETosis classically occurs following stimulation by PMA through activation of protein kinase C and the raf–mitogen-activated protein kinase (MEK)–extracellular signal-regulated kinase (ERK) pathway. NADPH assists in the translocation of neutrophil elastase from cytosolic granules into the nucleus where it aids in chromatin breakdown via histone cleavage. MPO is required for chromatin and nuclear envelope breakdown and granular mixing within the NET vacuole. Following 120 minutes of intracellular NET formation, the neutrophil outer membrane ruptures, and the mature NET is extruded. (D-F) By contrast, vital NETosis has been reported following both direct microbial exposure and lipopolysaccharide (LPS). Live S aureus induce rapid NET release (<30 minutes) in human and mouse neutrophils in vitro and in vivo. For gram-negative bacteria, NETs are induced via Toll-like receptor (TLR) 4 activation of platelets followed by direct neutrophil-platelet interaction via CD11a, whereas both complement receptor 3 and TLR2 are required for vital NETosis following gram-positive infection. NETs are released via nuclear budding (G-H) and vesicular release of NETs (I). This mechanism spares the PMN outer membrane, thereby allowing the PMN to continue to function, even to the point of becoming anuclear. (Panels G-I were reproduced from Pilsczek et al14 with permission (© 2010 by The American Association of Immunologists).
Suicidal NETosis vs vital NETosis. (A-C) Suicidal NETosis classically occurs following stimulation by PMA through activation of protein kinase C and the raf–mitogen-activated protein kinase (MEK)–extracellular signal-regulated kinase (ERK) pathway. NADPH assists in the translocation of neutrophil elastase from cytosolic granules into the nucleus where it aids in chromatin breakdown via histone cleavage. MPO is required for chromatin and nuclear envelope breakdown and granular mixing within the NET vacuole. Following 120 minutes of intracellular NET formation, the neutrophil outer membrane ruptures, and the mature NET is extruded. (D-F) By contrast, vital NETosis has been reported following both direct microbial exposure and lipopolysaccharide (LPS). Live S aureus induce rapid NET release (<30 minutes) in human and mouse neutrophils in vitro and in vivo. For gram-negative bacteria, NETs are induced via Toll-like receptor (TLR) 4 activation of platelets followed by direct neutrophil-platelet interaction via CD11a, whereas both complement receptor 3 and TLR2 are required for vital NETosis following gram-positive infection. NETs are released via nuclear budding (G-H) and vesicular release of NETs (I). This mechanism spares the PMN outer membrane, thereby allowing the PMN to continue to function, even to the point of becoming anuclear. (Panels G-I were reproduced from Pilsczek et al14 with permission (© 2010 by The American Association of Immunologists).
The cellular mechanisms that mediate lytic-NET release are still being worked out. For instance, lytic NETosis requires raf-MEK-ERK activation of NADPH oxidase,7 but it is worth noting that little is currently known about the signaling downstream of oxidants and upstream of NETs. In fact, it remains unclear how oxidants participate in the dismantling of the nuclear envelope and mixing of the NET components. Recent data link reactive oxygen species to NET release, through a neutrophil elastase (NE)–mediated mechanism, whereby NE translocates from cytoplasmic granules to the nucleus and instigates chromatin degradation through histone cleavage.8 Myeloperoxidase (MPO) also contributes to the decondensation of nuclear DNA, although the mechanism is not evident because the process does not require the enzymatic function of MPO. Clearly, many questions concerning the mechanisms of suicidal NETosis remain unanswered.
In spite of the early, very convincing work showing that PMA could not induce NETs in the absence of oxidants, and that humans with NADPH-oxidase deficiency could not make NETs,9-13 there is now growing evidence to suggest that some stimuli induce NETs independently of NADPH oxidase. Indeed, Staphylococcus aureus appears to induce NETs in a rapid fashion before any intracellular oxidative stress could be detected.14 Additionally, hydroxymethylglutaryl–coenzyme A reductase inhibitors, which are used to lower cholesterol in humans, block PMN oxidative burst, leading to enhanced NETosis against staphylococci.15 Winterbourn and colleagues have expanded on the research in this area by demonstrating that certain NET-inducing stimuli are oxidant dependent, whereas others are not.16 Similarly, fungi, such as Aspergillus, can also induce oxidant-independent NET release.17 Clearly, the absolute requirement of oxidants for NET formation may be disease specific and not universal to all forms of NETosis.
A key requirement of NET generation is the mobilization of nuclear material from the nucleus to the environment. Many eukaryotic cells routinely dismantle their nuclear envelope, independent of NADPH oxidase, in order to divide DNA. Therefore, molecular insights into how PMNs free DNA from the nucleus may be gained by reviewing how other cells undergo nuclear division.
The nuclear envelope consists of an outer and inner lipid membrane and aqueous pore complexes structurally held together by intermediate protein filaments called lamina, which can be torn open by microtubules. Breakdown of the nuclear envelope can be rapid, as demonstrated by the biphasic mechanisms observed in oocytes. Here, the pore complex partially disassembles in 10 minutes (phase 1), followed by complete envelope permeabilization within 35 seconds (phase 2) and finally complete lamina dissociation, a process requiring microtubules18 and an additional 10 minutes.19 It is unclear if these mechanisms also occur in PMNs or if they are required for liberation of DNA into the cytoplasm prior to NET release. Certainly, PMNs are likely to employ unique mechanisms of nuclear envelope breakdown. For example, elastase relocation to the nucleus is a prerequisite for NET release, yet elastase is, for the most part, restricted to the neutrophil. In addition, PMNs have very unique nuclear architecture characterized by a multilobar appearance and a paucity of known nuclear envelope proteins.20 Specifically, neutrophils are devoid of the molecules that have been implicated in cellular division including linker of nucleoskeleton and cytoskeleton complex proteins: nesprin 1 giant, nesprin 2 giant, SUN1, and plectin.21
Epigenetic mechanisms may also play a role in NET release. Wang et al found that histones modified by citrullination were required for NETosis and that inhibition of PAD4, the enzyme required for citrullinating proteins in neutrophils, disabled NET formation.22 Mice with PAD4 deficiency displayed impaired NETosis and were highly susceptible to some severe skin infections such as necrotizing fasciitis.23 However, it is equally important to point out that PAD4 deficiency does not contribute, for example, to lung infections, cause by influenza virus,24 despite reports demonstrating severe inflammation caused by influenza-induced pulmonary NETosis.25 Although citrullination of histones has been a growing area of interest in the arthritis field and evidence suggests that they are the source of autoantigens,26,27 it is worth noting that targeting PAD4 has had divergent results in ameliorating autoimmune disease. For instance, PAD4 deficiency did not affect the development of arthritis in mice, despite inhibiting the ability of PMNs to release NETs within the joint.28 On the other hand, a well-recognized general chemical inhibitor of all PADs (Cl-amidine) did inhibit tissue injury associated with mouse models of arthritis as well as inflammatory bowel disease.29,30 However, whether NET formation was associated with PAD4 inhibition or some unrelated PAD mechanism remains unclear because these studies did not examine or consider NETs. A new publication directly demonstrated that PAD chemical inhibition (Cl-amidine) inhibited NETosis in lupus-prone mice leading to reduced autoantibodies, decreased antibody deposition in the kidney, improved endothelial function, and diminished disease activity.31 Why differences exist between chemical inhibition and PAD genetic deficiency is not clear; however, Cl-amidine inhibits other PADs that may be involved in other aspects of disease progression.
Neutrophil lysis
A defining component of suicidal NETosis is the requirement of plasma membrane rupture and cell death. These defining features are shared between NETosis and pathogen-induced immune cell lysis, resulting in divergent theories and controversy. On one hand, lytic NETosis is thought to be a beneficial immune response responsible for capturing pathogens; whereas on the other hand, pathogen-induced lytic cell death is considered to be a pathologic immune evasion strategy. To provide balance to the previous discussion about lytic NETosis, we will outline recent advances in pathogen-mediated host cell killing.
Pathogen-mediated cell lysis is a well-established microbial mechanism of immune evasion and shares multiple similarities with lytic NETosis. S aureus and Streptococcus pyogenes produce hemolytic and leukocytic proteins capable of either red blood cell (RBC) lysis or leukocyte rupture. S aureus effectively and rapidly kills isolated human neutrophils in vitro.32 These bacteria can produce bicomponent leukotoxins, which are pore-forming lytic enzymes that attack the leukocyte plasma membrane resulting in hypoosmotic cell lysis. Several groups of these enzymes have been described, including γ-hemolysin, leukocidin E/D, the bacteriophage-encoded Panton-Valentine leukocidin, the leukocidin M/F-PV-like, and the recently described LukGH.33-38 Additionally, phagocytosed S aureus can escape phagolysosomal killing by attacking the neutrophil from the inside out. DeLeo and colleagues observed that S aureus, and particularly community-acquired methicillin-resistant S aureus, could kill PMNs within 4 to 6 hours of being ingested. Interestingly, the neutrophil deaths had features of apoptosis; however, these cells were unable to maintain plasma membrane integrity and died by lysis, which is not consistent with normal apoptosis. Conversely, PMNs were also observed to die by true apoptotic mechanisms without lysing.32 S aureus did not directly perforate the PMNs using toxins but instead initiated the upregulation of calcium regulatory transcripts leading to a programmed necrosis. Additionally, S aureus can upregulate phenol-soluble modulins, leading to host cell lysis, in reaction to being phagocytosed.39 This process occurs hours after bacteria are ingested, and as a result, the pathogens ultimately escape their intracellular prison.
The key issue is that although NETosis and overt cell death induced by pathogens may represent unique processes, differentiating and defining them remains complex. Further research is required to fully compare and contrast these processes and to establish unique molecular characteristics of both NET DNA and lytic DNA. For example, citrullination of histones and the presence of proteases, normally confined to intracellular granules, deposited on chromatin may distinguish NETosis from other forms of cell death. Additional characterization of NETosis will, ideally, allow researchers to specifically target and inhibit the process, thereby improving the ability to distinguish NETosis from pathogen-induced lysis. Of note, we do not believe that simply assaying for cell-free DNA should be accepted as sufficient evidence that NETosis, and no other form of lytic cell death, has occurred.
Vital NETosis
Suicidal NETosis is cellular kamikaze, requiring membrane rupture and the loss of conventional live neutrophil functions, such as leukocyte recruitment, chemotaxis, and phagocytosis. Prior to the discovery of suicidal host defense, the established classical paradigms of neutrophil-mediated host defense and inflammation40,41 involved intact live neutrophils (Figure 1). How these opposing cellular strategies, one requiring live cells and the other causing cell death, are both essential for adequate host defense is controversial. One possibility is that NETosis may occur in the absence of cellular suicide, thereby allowing normal functions to continue. Supporting this idea, our group has uncovered a nonsuicidal pathway of NETosis.14,40,41 This pathway, termed vital NETosis, allows NET release and conventional host defense to coexist. Subsequently, we compare and contrast “vital NETosis” with suicidal NETosis.
Clearly a lysed, dead neutrophil could not undergo leukocyte recruitment, chemotaxis, and phagocytosis. Potentially, however, a PMN could first perform its live cell functions and subsequently undergo suicidal cell death; nevertheless, cell lysis after pathogen capture would free imprisoned bacteria. Therefore, a sequential model of live cell functions followed by suicidal cell functions does not seem likely. Thus, other explanations may exist, including the hypothesis that there are subsets of neutrophils, in which one subset primarily performs live cell functions, such as phagocytosis and containment, whereas another subset is responsible for suicide and NETosis. This would imply that conventional host defense and NETosis are mutually exclusive. To date, limited evidence exists to support this idea. Alternatively, the possibility exists that neutrophils survive the NETosis process and continue to perform the functions necessary to detect, capture, and contain pathogens. It is worth noting that this mechanism could still be consistent with the possibility that a specialized subset of neutrophils performs NETosis because it has been well documented that at most 20% to 25% of PMNs release NETs.
Numerous groups have put forth the idea that an alternative NETosis pathway exists in addition to the lytic–cell death pathway (Figure 2). Clark et al demonstrated the release of NETs from an intact neutrophil that continued to restrict intracellular access of SYTOX Green, supporting the idea that PMNs can remain intact.40 Additionally, Yousefi and colleagues, first in eosinophils but subsequently in neutrophils, described a form of NETosis that caused a catapult of the DNA from mitochondria without causing lytic cellular death.42,43 Conversely, a new report describes eosinophil trap formation resulting in cytolytic granular release.44 Therefore, granulocytes in general may have both a lytic and nonlytic pathway of NETosis.
The first fundamental differences between suicidal NETosis and vital NETosis are the nature of the inciting stimuli and the timing of NET release. For instance, suicidal NETosis has mostly been demonstrated in the context of PMA chemical stimulation. As previously noted, this pathway of NETosis requires hours. In contrast, vital NETosis has been demonstrated following microbial-specific molecular patterns recognized by host pattern recognition receptors. In particular, LPS, a gram-negative bacterial stimulus, induces rapid NET release.40 This rapid NETosis did not involve cell lysis and was specifically mediated by TLR4 on platelets that facilitated activation of PMNs. Moreover, activation of the vital NETosis pathway appears to be a generalized response against various classes of microbial pathogens. Indeed, S aureus induces human PMNs to rapidly release NETs via nuclear envelope blebbing and vesicular exportation, thereby preserving the integrity of the PMN plasma membrane (Figure 2).14 In vivo, rapid NETosis following live gram-positive bacteria was mediated by both TLR2 and complement.41 Furthermore, rapid microbial-induced NETosis is not limited to bacteria, because a recent report found that Candida albicans stimulated NETosis within 30 minutes in a fibronectin- and complement-dependent manner.17
A second major defining difference between suicidal NETosis and vital NETosis depends on the functional capacity of the PMNs during NET release. Initial in vitro studies documented that microbial-induced NETosis spared the PMNs from lysis; however, these experiments could not address the functional capacity of the NETosing PMNs. To directly investigate if NETosis could be carried out by live functional PMNs, our laboratory developed a method to directly visualize NETosis in a mouse model of a bacterial skin infection using intravital confocal microscopy. Here PMNs rapidly released NETs within the skin in a TLR2- and complement-mediated pathway and maintained the ability to chemotax and phagocytose live bacteria. During the initial acute response, neither PMN lysis nor evidence of cell death was apparent; however, NETosing neutrophils became anuclear cytoplasts capable of chasing and imprisoning live Staphylococcus.41 Hence, we revealed a major fundamental difference between vital NETosis and suicidal NETosis. Recent data corroborate our findings in that PMNs remain motile and release NETs via a vesicular mechanism when contacting C albicans.17 Here, NET formation also recruited additional PMNs in a swarming-like behavior.
The third fundamental difference between suicidal NETosis and vital NETosis involves the mechanisms employed to make and release NETs. As previously detailed, suicidal NETosis requires PMA stimulation of raf-MEK-ERK and subsequent activation of NADPH oxidase.7 MPO and elastase mediate the decondensation of chromatin resulting in a mixture of DNA and granule proteins that are extruded out of a perforation in the plasma membrane. In contrast, vital NETosis requires vesicular trafficking of DNA from within the nucleus to the extracellular space. Using electron microscopy, our laboratory demonstrated that vesicles of DNA budded from the nuclear envelope, passed through the cytoplasm, and coalesced with the plasma membrane, thereby delivering the NET out of the cell without requiring membrane perforation.14
Anuclear cellular function
Critics of vital NETosis argue that a cell, like a PMN, could not live and function without an intact nucleus; however, multiple well-established examples of anuclear cell function exist. All eukaryotic cells begin their life with a nucleus, although clear examples demonstrate that cells can not only survive the process of enucleation, but also function without a nucleus. For example, progenitor RBCs form in the bone marrow with a nucleus, and as they mature they lose the entire nuclear structure, prior to entering the blood circulation. RBC enucleation is a multistep process involving the cytoskeleton, signaling pathways, and formation of a contractile actin ring via Rac guanosine triphosphatases and mDia2.45-47 The maturing erythrocyte requires the protein survivin, an inhibitor of apoptosis, which mediates endocytic vesicle trafficking.48 Interestingly, overexpression of survivin in cultured cells results in enucleation. Despite not possessing a nucleus, the mature RBC remains metabolically active and survives in the circulation for 120 days. Immature neutrophils also express survivin, which is downregulated during maturation but can be induced using growth factor such as granulocyte macrophage–colony-stimulating factor.49 It is not known if survivin expression enhances NETosis or if immature PMNs are the primary NETosing cells due to expression of this protein.
Platelets are derived from megakaryocytes, which are nucleated cells found in the bone marrow. Small pieces of the mature megakaryocyte are sheared from the plasma membrane, thereby forming mature anuclear platelets.50,51 Platelets survive in the circulation for approximately 7 days and carry out multiple functions, during hemostasis, immunity, and inflammation including phagocytosis, chemotaxis, and pathogen killing. Interestingly, these anuclear cells carry preselected messenger RNA that can be translated to protein and even have the capacity to divide within the circulation.52-54
Functional anuclear granulocytes can be generated as either cytoplasts or motile cytokineplasts.55,56 Applying heat to human neutrophils generates nuclear-free and granule-depleted PMN remnants capable of chemotaxis toward fMet-Leu-Phe. Importantly, these cytoplasts can adhere to endothelium and transmigrate across a human endothelial monolayer57 as well as home to inflammatory sites in vivo.58 Centrifugation of PMNs forms anuclear cytoplasts,59 which are not only cryopreservable but also retain the ability to chemotax and phagocytose bacteria such as S aureus60 and kill them.61 Therefore, considering that the neutrophil half-life is only 24 to 48 hours, the concept of anuclear PMN survival is not impossible. The intriguing question here is, If there are anuclear neutrophils, how are these cytoplast-like cells ultimately eliminated?
NETosis and host defense
The most contentious issue in this field is no longer the existence of NETs, but rather their exact function. The exact mechanisms by which NETs mediate host defense require further investigation, and discordant opinions exist. It is our contention that more sophisticated imaging will be needed with appropriate vital dyes to delineate whether NETs trap and/or kill microbes. Here 2 fundamental aspects of NET function are presented pertaining to host defense: (1) trapping and capture; and (2) direct microcidal activity.
Trapping
NETs have been proposed to have a number of antimicrobial effects, including the ability to physically adhere to microbial pathogens, thereby trapping them. Direct imaging evidence for microbial capture exists for a number of different pathogens. The initial description of NETs demonstrated, using electron microscopy and immunofluorescence, the ability of extracellular nucleic acids to adhere to exogenously added S aureus, Salmonella typhimurium, and Shigella flexneri post-NETosis stimulation.4 Furthermore, in a model of rabbit shigellosis, bacteria were found adhered to the NET structure. In total, multiple bacteria have been shown to bind directly to extracellular DNA in vitro, including Streptococcus pneumoniae, S aureus, and Escherichia coli.14,62-64 In vivo, Klebsiella pneumoniae has been visualized trapped within NETs in the lungs of infected mice.8 Clearly, substantial evidence exists demonstrating that bacteria can stick to extracellular DNA; however, these highlighted imaging experiments do not definitively establish that NETs are trapping bacteria. Conceivably, these experiments could be highlighting that bacteria take advantage of the sticky DNA to establish an attachment point. In fact, biofilm-forming bacteria like Pseudomonas aeruginosa use DNA to build biofilms and even digest extracellular DNA for nutrients. Supporting the trapping hypothesis, our laboratory used a flow chamber system, in vitro, to demonstrate that E coli could be ensnared by NETs under physiological shear forces compatible with blood flow. Additionally, liver intravital microscopy revealed that E coli was captured in vivo by NETs.40 Both the in vitro and the in vivo NET formation occurred through a platelet TLR4-dependent mechanism.
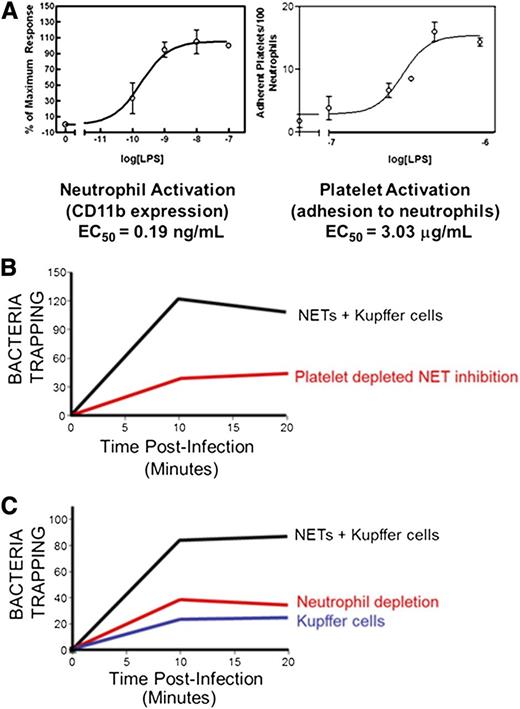
A more detailed assessment of NET trapping using spinning disk confocal intravital microscopy of the liver during endotoxemia found that the release of NETs into hepatic sinusoids resulted in the capture of circulating E coli and the prevention of dissemination65 (Figure 3). Here, the platelet-neutrophil interaction leading to NET formation required the integrin LFA-1 because inhibition of this molecule prevented NET formation. Previous studies, prior to the discovery of NETs, attributed the capture of circulating bacteria exclusively to the liver sinusoidal macrophages, or Kupffer cells, via expression of the complement receptor CRIg.66 However, our data suggest that NET release increases total bacteria capture by three- to fourfold, independent of macrophages.
Intravascular bacteria are optimally captured via platelet-induced NET release and resident vascular macrophages. (A) Platelet activation is essential for in vivo NETosis. Isolated neutrophils become activated at very low levels of LPS; however, platelet activation occurs at significantly higher LPS concentrations, suggesting that platelet activation acts as an inflammatory barometer to maximally stimulate neutrophils during periods of severe infection. (B) In vivo, Kupffer cells are considered the major cell involved in intravascular bacterial capture. However, under basal conditions, when NETs are not yet present, Kupffer cells only account for a third of the microbial trapping. Similarly, under LPS-stimulated conditions, depletion of PMNs resulting in NET deficiency yields levels of capture similar to Kupffer cells alone. Hence, optimal bacterial capture occurs when Kupffer cells, PMNs, and NETs are present. (C) In vivo, during endotoxemia, platelet depletion diminishes neutrophil activation and NET release, thereby impairing optimal bacterial capture. EC50, 50% effective concentration.
Intravascular bacteria are optimally captured via platelet-induced NET release and resident vascular macrophages. (A) Platelet activation is essential for in vivo NETosis. Isolated neutrophils become activated at very low levels of LPS; however, platelet activation occurs at significantly higher LPS concentrations, suggesting that platelet activation acts as an inflammatory barometer to maximally stimulate neutrophils during periods of severe infection. (B) In vivo, Kupffer cells are considered the major cell involved in intravascular bacterial capture. However, under basal conditions, when NETs are not yet present, Kupffer cells only account for a third of the microbial trapping. Similarly, under LPS-stimulated conditions, depletion of PMNs resulting in NET deficiency yields levels of capture similar to Kupffer cells alone. Hence, optimal bacterial capture occurs when Kupffer cells, PMNs, and NETs are present. (C) In vivo, during endotoxemia, platelet depletion diminishes neutrophil activation and NET release, thereby impairing optimal bacterial capture. EC50, 50% effective concentration.
Indirect, nonimaging experiments using bacterial culture and dissemination models support the role of NETs in trapping bacteria. We previously demonstrated, using whole animal luminescent imaging, that administering systemic DNase during Staphylococcus cellulitis resulted in rapid bacterial escape of the bacteria from the inoculation site. Rapid escape following NET breakdown increased the level of blood-borne pathogens, thereby confirming that the DNA backbone is critical for containing infection.41 Similarly, many bacteria produce nucleases, and experimental evidence suggest that these enzymes increase bacterial invasiveness. For instance, group A Streptococcus (GAS) causes life-threatening diseases in humans, including sepsis and necrotizing fasciitis, and it expresses nucleases. Inhibiting the function of DNase enzymes encoded by either SdaD2 or Sda1 within GAS through mutation significantly increases PMN-mediated clearance and reduces tissue necrosis.67,68 Conversely, inducing nonnuclease GAS to express Sda1-encoded DNase inadvertently causes a hypervirulent form of Streptococcus through the Sda1-selected mutation of the virulence regulatory sensor kinase (covRS) operon.69 Similar observations have been demonstrated for both S pneumonia and S aureus. For S pneumoniae, endonucleases are inducible and allow bacteria to escape NET trapping resulting in dissemination from the lung into the blood.62 In addition, encapsulation of pneumococcus provides resistance to NET trapping, potentially via electrochemical repulsion.63 For S aureus, nuclease expression during pneumonia impairs bacterial clearance from the mouse lung resulting in increased mortality.70
Disseminated fungal infections are common in immunocompromised neutropenic humans. Consistent with this observation, NETs can trap C albicans.17,71 Trapping these pathogens is important because phagocytosis is inefficient. Using scanning electron microscopy and confocal microscopy, Zychlinsky et al demonstrated that C albicans initiates and adheres to the NET structure.71 Aspergillus fumigatus has also been visualized being captured by NETs using immunofluorescence and scanning electron microscopy, and within ex vivo lung tissue using 2-photon microscopy.72,73
The mechanisms of neutrophil-mediated antiviral activity remain undefined, although new evidence suggests that viruses can stimulate NET release.24,25,74,75 Recently, superresolution structured illumination microscopy directly demonstrated that HIV elicits NETs through PMN-expressed TLR7 and TLR8. Here, HIV particles attached to NETs and were liberated following DNase treatment.74 Importantly, CD4+ T cells were protected from HIV infection if PMNs were cocultured. In our laboratory, intravital microscopy showed that virus-activated TLR3 induces platelet-neutrophil interaction within the vasculature leading to increased NET formation and a reduced number of infected hepatocytes.76
How NETs effectively bind and capture such a huge range of different microbes is not clear. It has been hypothesized that nucleic acid charge is important; however, more specific interactions may also exist. In particular, surfactant protein D, a C-type lectin-containing pattern recognition receptor, binds both bacterial pathogens and NETs, thereby forming an intermediary bridge.77 In vivo, emigrated neutrophils release NETs that spread to cover a large tissue area. These tissue PMNs dragged NETs as they crawled, thereby forming an effective trapping structure that prevented bacterial dissemination into the vasculature and perhaps the lymphatics. A potential explanation for the tissue-wide NET distribution is the fact that fibronectin, highly expressed in the interstitium, has a DNA-binding site. This may also explain why in tissues, in vivo, one sees large sheets of DNA, whereas in vitro they often appear as thin strands. On the other hand, Saitoh et al also visualized large sheets of DNA in vitro using superresolution microscopy.74
Direct NET antimicrobial activity
Without a doubt, there is much debate over whether NETs can kill bacteria directly, beyond just capturing and immobilization. Proteases, antimicrobial molecules and histones, as well as DNA, are part of NETs, and as such it is tempting to conclude that these structures can directly kill microbes. In fact, the antibacterial properties of histones have been recognized for decades78 ; however, how these proteins kill and under what circumstances they would be found outside of the nucleus were not known. Traditionally, phagocytosis is required for killing by neutrophils, but when PMNs are treated with cytochalasin D, to inhibit phagocytosis, they retain bactericidal activities that are eliminated by either exogenous DNase or antihistone antibodies against H2A.4 Therefore, it would appear that NETs have some ability to directly kill microbes. Additionally, antimicrobial activity has been demonstrated against a number of pathogenic organisms,9,14,68,71 although the key molecules and mechanisms remain elusive and undefined. It is known that certain host enzymes such as neutrophil elastase can neutralize pathogen virulence factors like those produced in Shigella.79 Given the finding of extracellular elastase decorating NETs, researchers found that NETs could inactivate extracellular bacterial virulence factors such as IpaB from S flexneri.4 Furthermore, blocking NE results in high levels of IpaB.
NETs may also serve to opsonize certain fungi such as A fumigatus via long pentraxin 3, which is thought to be an ancient ancestral antibody.80 NETs generated from PMNs via the chemical PMA can inhibit the growth of Aspergillus,9 possibly through zinc chelation via calprotectin.67,76 C albicans can also be killed by PMA-induced NETs. This mechanism involved DNA-dependent killing and calprotectin, but not histones.71,81 NET chelation of cations may also contribute to the killing of the opportunistic pathogen P aeruginosa.82 Whereas NETs are sufficient to kill Pseudomonas grown in suspension, during diseases such as cystic fibrosis, the bacteria changes to a mucoid type and can become NET resistant.83
By contrast, other groups have not been able to demonstrate significant direct killing by NETs. In the case of S aureus, despite clear trapping by the NETs, DNase treatment liberated living bacteria that were capable of proliferation.84 The addition of toxic antimicrobial granular proteases to the DNA backbone is one hypothesis of how NETs can directly kill. However, in the late 1980s and early 1990s, a series of papers nicely demonstrated that for proteases to be active, neutrophils formed a sequestered space between themselves and the underlying structure, thereby excluding ubiquitous and abundant antiproteolytic enzymes.85-88 Extracellular release of elastase and other proteases had to occur into this sequestered space, otherwise the proteolytic activity was rapidly inactivated by plasma. In fact, 1 mL of plasma has sufficient α1-proteinase inhibitor activity to inactivate the elastase of 500 million neutrophils.89 Therefore, it remains unclear how elastase released in the form of an NET can possibly retain its proteolytic, and microcidal, activity, unless it is in some way protected by the DNA structure. Many in vitro studies avoid the use of plasma when studying the killing capacity of NETs, and this could be an important factor. Because there are no direct inhibitors of NET production, without impairing other neutrophil mechanisms (eg, oxidant production), it is not easy to conclude how important NETs are to killing. In addition, blocking phagocytosis also suffers from similar nonspecific limitations, making it difficult to determine how much killing occurs via NETosis vs phagocytosis.
It is worth mentioning that because of their histone components, NETs have the capacity to cause bystander injury. Extracellular histone proteins can activate TLR and lead to the generation of thrombin as well as activate platelets resulting in microaggregation and thrombocytopenia.90-92 Furthermore, treating neutrophils with LPS-activated platelets induces NETs and leads to the killing of endothelium.40 Although the mechanism of killing was not elucidated in that study, subsequent work has shown that histones are potent cytotoxic molecules for endothelium.93-95 Both these groups have postulated that this histone-induced NET injury to the vasculature could be a major contributor to the multiple organ dysfunction observed in sepsis.
A related and emerging area of NET research is the relationship between NETs and thrombosis. The concept that tissue injury, inflammation, and infection increase the risk of venous thrombosis is well established. However, the underlying mechanisms have not been clearly defined. Insight into the relationship between NETs and clotting began with the observation that extracellular histones induced microthrombosis. In addition, it was found that protein C, a physiological anticoagulant, specifically cleaved extracellular histones from NETs, thereby reducing their toxic vascular effects.93 Currently, researchers are defining how NETs promote thrombosis96,97 and developing new concepts such as immunothrombosis, which is the idea that innate vascular host defense and clot formation are intimately linked, partly through the release of NETs.98-100
Autoimmune and vasculitic disease
The underlying trigger or inciting cause of autoimmune diseases and vasculitides is largely unknown; however, the role of infection has long been proposed.101-103 Therefore, NET release during acute infection may have unintended, long-term side effects that must be considered. Here we consider the evidence that NET release may incite both autoimmune and vasculitic diseases.
Anti-neutrophil cytoplasmic antibodies (ANCAs) are autoantibodies directed against intracellular neutrophil antigens. These antibodies help define and diagnose vasculitis, although it remains uncertain if they are pathogenic or merely associated with disease. ANCA-associated vasculitis often begins with either pulmonary and/or renal dysfunction. Three main disease subsets have been characterized by the immunofluorescent staining pattern produced by the abnormal autoantibodies on normal PMNs. Serum from patients with Wegener granulomatosis produces a diffuse granular cytoplasmic staining pattern due to proteinase 3 (PR3) autoantibodies, whereas serum from patients with microscopic polyangiitis and Churg-Strauss demonstrate a perinuclear staining pattern due to anti-MPO autoantibodies. Both PR3 and MPO are components of NETs. Two fundamental questions have remained insufficiently answered for these diseases: (1) How do autoantibodies directed against intracellular PMN antigens form? (2) Are ANCA antibodies a marker of disease, or do they participate in pathogenesis?
The molecular mimicry theory suggests that certain bacterial species contain antigens sufficiently similar to the host antigens PR3 and MPO to induce autoantibodies and possibly disease. On the other hand, PMN cytoplasmic antigen release, because of host defense mechanisms, may be sufficient to trigger autoimmunity without the need of microbial mimicry. Epidemiological studies have shown that humans with Wegener granulomatosis are at greater risk of disease relapses if they have a high burden of S aureus nasal carriage.104 Indeed, Kessenbrock et al revealed that PMN-mediated host defense and the release of NETs is sufficient to induce small-vessel vasculitis.105 Although nucleosomes were previously known to circulate in vasculitis patients,106 Kessenbrock directly demonstrated that the circulating DNA was attached to MPO, an indicator of authentic NETs, and deposited within the microvasculature of patients with vasculitis. Furthermore, it was demonstrated that ANCA could actually stimulate the release of further NETs, thereby establishing a feed-forward autoimmune cycle. This self-propagating disease process is driven by ANCA binding to either immunoglobulin G or immunoglobulin A Fc receptors on neutrophils.107 Interestingly, vasculitis can be initiated by exposing myeloid dendritic cells to NETs, allowing antigen processing, and adoptively transferring the stimulated myeloid dendritic cells into a naive mouse.108 Finally, the human thyroid medication propylthiouracil is associated with causing ANCA production and ANCA-associated vasculitis. A recent study demonstrated that propylthiouracil given to NETosing PMNs resulted in disordered NETs, which were resistant to DNase and had the ability to induce vasculitis in rodents.109
Systemic lupus erythematosus (SLE, lupus) is a complex autoimmune disease, comprising a constellation of clinical manifestations of unknown cause. In addition to important clinical features, serological testing often reveals high levels of antinuclear antibodies and anti–double-stranded DNA antibodies. Similar to the ANCA-associated diseases, it is not clear how or why antinuclear antibodies or double-stranded DNA antibodies form or if they are pathogenic; however, the potential of circulating NETs causing autoimmune disease is an enticing hypothesis.
A review of the historical diagnostic tests for lupus provides intriguing, although inconclusive, evidence that NETosis may play a pathophysiological role in SLE. For instance, lupus erythematosus cells (LE cells) and tart cells were first reported in 1948 by Hargraves and Morton.110,111 The tart cell was characterized by having a “secondary nucleus” that was partially digested DNA often appearing partly outside of the cell membrane. Because the formation of the tart cell has never been directly visualized, it is not clear if this cell is potentially extruding or phagocytosing the nuclear material. In other words, the tart cell may either be in the process of making an NET or digesting one. Additionally, the LE cell is thought to represent a PMN that has phagocytosed a cell-free nucleus and also contains cytoplasmic vacuoles with partially digested nuclear material, although the source of this cell-free nucleus and circulating DNA has never been established. Alternatively, it has been hypothesized that the LE cell represents autolysis of one of the lobes of the PMN nucleus. Interestingly, Hargraves took lupus patient serum and added it to normal bone marrow, which resulted in LE cell formation and the observation of neutrophil clusters surrounding extracellular nuclear material, analogous to the ability of serum ANCA to induce NETs. LE formation has also never been directly observed. Therefore, it is not clear where the extracellular DNA came from and whether the vacuoles filled with digested DNA are being released by the PMN or being phagocytosed. It also remains possible that tart cells and LE cells may actually represent different forms of NETosis, in other words lytic NETosis and vital NETosis.
The pathogenic process leading to organ failure and death in SLE is complex and not fully defined; however, the presence of circulating nucleic acids, nucleosomes, and autoantibodies directed against these components has long been recognized.112-115 Subsequently, we will discuss the relationship between homeostatic enzymatic degradation of extracellular DNA and the development of SLE and lupus kidney dysfunction known as lupus nephritis.
Animals express multiple enzymes for the digestion of intracellular and extracellular nucleic acids, and these enzymes, in particular DNase1, have been examined for their role in autoimmune diseases. Mice genetically deficient in DNase1 develop a syndrome similar to human lupus,116 and humans with lupus have been found to have mutations within the DNase1 gene.117 Moreover, evidence exists demonstrating the importance of DNase1 in the development of lupus nephritis, a disease in which cell-free nucleosomes, chromatin, and autoantibodies are deposited within the kidney glomerular basement and capillary membranes.118 In lupus-prone (NZBxNZW) F1 mice, kidney injury develops secondary to extracellular nucleosome deposition within the kidney, although the origin of the nuclear material was not clear because apoptotic programmed cell death was not the source.119 Nucleosome-induced injury was further compounded by the presence of underlying anti-DNA autoantibodies that promoted an acquired reduction in DNase1 expression within the kidney, ultimately resulting in accumulation of undigested extracellular chromatin.119,120 Despite this evidence supporting a role of DNase in lupus pathogenesis, these investigations did not directly link DNase and NETosis to the development of SLE.
The first direct evidence implicating NETs in the pathogenesis of lupus came in 2010 by the Zychlinsky laboratory.121 The authors proposed that compared with healthy humans, lupus patients may have a diminished ability to degrade NETs. Supporting this hypothesis, normal human serum rapidly degraded NET structures. Furthermore, the addition of chelating divalent cations, such as calcium, or exogenous G-actin, inhibited serum-induced NET degradation, both of which are known to block the function of DNase1.122,123 Moreover, inhibitory anti-DNase1 antibodies blocked NET degradation. Interestingly, a subset of lupus patients was found to have impaired NET degradation, and their serum was termed “nondegraders.” Two different mechanisms explained the NET degradation impairment: (1) a group of patients harbored a serum DNase1 inhibitor; or (2) they had developed anti-NET antibodies that had the capacity to block access of the DNase1 enzyme to the DNA backbone, thereby protecting the NET from degradation. These mechanisms were independent of the reported DNase1 genetic mutation found in a small group of patients. Finally, they revealed that SLE nondegraders were at higher risk of developing nephritis and had pathological evidence of NET deposition within the kidney.121
Further investigation has revealed that subsets of neutrophils, expanded in lupus patients, are prone to NETosis, potentially due to overexpression of the NET-dependent proteins MPO and elastase.124 NET components subsequently damage the vasculature, activate plasmacytoid dendritic cells via TLR9 leading to interferon release, and ultimately serve as pathogenic autoantigens.124-126 During SLE flairs, patients have a reduced ability to clear NETs, possibly due to C1q inhibition of DNase, resulting in persistent complement activation and continued disease.127 Additionally, macrophages from lupus patients are highly sensitive to NET-induced activation through the NLRP3 inflammasome, thereby contributing to a feed-forward proinflammatory response, further compounding disease progression and severity.128
Rheumatoid arthritis (RA) is an autoimmune disease that targets joints, but it can also cause severe systemic and solid organ complications. The generation of autoantibodies to citrullinated protein antigens is thought to play an important role; however, how the immune system generates autoantibodies to these intracellular targets is unclear. PMNs from RA patients were found to be more prone to produce NETs, and RA serum was a strong inducer of NETosis. Moreover, NETosis resulted in the externalization of citrullinated autoantigens, thereby linking NET formation to RA.27
Conclusions
As with any new discovery, there is much enthusiasm about NETs and their potential to explain many different disease processes. A number of these may withstand the test of time, whereas others may not. Purported NETosis stimuli have included the ubiquitously made chemokine IL-8, oxidants, LPS, and many other molecules. However, because NETs are indeed quite toxic to the host, it would seem inconceivable that these structures would be released anytime a small amount of LPS was encountered or IL-8 was produced. Indeed, in the case of platelet-induced neutrophil NET production, the neutrophil activates at an LPS EC50 of 0.19 ng/mL but does not make NETs; whereas platelet activation required an LPS EC50 of 3.03 µg/mL (Figure 3). This suggests that there is a safeguard that prevents neutrophils from making NETs in the vasculature during, for example, endotoxemia unless LPS concentrations reach sufficient levels (suggesting severe infection) to activate platelets. Therefore, the platelet is the inflammatory barometer that activates the neutrophils, following sufficiently potent stimuli, to finally make NETs. It is worth noting that plasma from septic patients induced NETs in the presence of platelets, and this was only partly TLR4 dependent, suggesting that LPS may contribute to NET formation but may not be the only NET inducer in sepsis.
The release of DNA has also been reported from mast cells, eosinophils, basophils, and more recently from macrophages.42,44,129-133 Although these events might occur, the evidence is less strong. In the case of eosinophils, the release of DNA covered with potent eosinophil-derived neurotoxin or major basic protein may be an important way of sequestering and directing dangerous molecules toward large parasites through a catapult-like mechanism. Indeed, the release of eosinophil-derived neurotoxin directly into the blood stream would not be a wise strategy, as was shown in the 1980s with eosinophil-stimulating molecules in health food tryptophan supplements that caused debilitating neurotoxicity.134
The idea that the eosinophil NETs are derived from mitochondria rather than nuclei is also interesting and may allow preservation of these cells after NET release. However, in the case of the neutrophils, the majority of studies suggest the NET DNA contains histones and is therefore of nuclear origin. It is interesting that there are far fewer examples of autoantibodies against mast cell, endothelial, or even eosinophil specific antigens relative to neutrophil autoantibodies, suggesting that DNA release from these cells is far less frequent than from neutrophils. The importance of DNA release from macrophages has only recently been reported, and this requires further exploration to understand the significance but could be an important mechanism in granuloma formation.129-131
A debate once raged around the very existence of DNA. Yet today, this debate would seem irrational and preposterous. Similarly, the debate around the existence of NETs is also slowly fading as more and more labs report the existence of these structures. The focus is shifting to what signaling pathways induce NETs and how these pathways can be inhibited or manipulated. Importantly, it remains unclear which diseases may benefit from inducing or enhancing NETosis and in which diseases NETosis should be inhibited. In addition, it is unclear how certain bacteria manage to fly under the radar and avoid NET induction or prevent NET release. It is worth noting that the mechanism of NET release has been identified in fish neutrophils, and even root nodular cells, suggesting that this program could be an evolutionarily conserved ancient immune mechanism.
In summary, we have provided evidence of a “vital NETosis” pathway distinct from suicidal NETosis, although further investigation is required to fully understand these processes. We believe that the evidence supports NET release as a vital host defense mechanism. Nonetheless, considerable knowledge gaps remain; therefore, continued investment into NETs research is essential.
Acknowledgments
This work was supported by operating grants from the Canadian Institute of Health Research. P.K. is an Alberta Innovates Health Solutions Scientist, Canada Research Chair, and the Snyder Chair in Critical Care Medicine. B.G.Y. is a Clinical Scholar in the Department of Critical Care Medicine (University of Calgary), an Alberta Innovates Health Solutions Clinical Fellow, and a Canadian Institute of Health Research Fellow.
Authorship
Contribution: B.G.Y. produced the figures and wrote the manuscript; and P.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bryan G. Yipp, HRIC 4AA16, University of Calgary, 3330 Hospital Dr N.W., Calgary, AB, T2N 4N1, Canada; e-mail: bgyipp@ucalgary.ca; and Paul Kubes, HRIC 4AA16, University of Calgary, 3330 Hospital Dr N.W., Calgary, AB, T2N 4N1, Canada; e-mail: pkubes@ucalgary.ca.