In this issue of Blood, Kasahara et al report that platelet-dependent clot retraction requires factor XIII (FXIII), which covalently associates fibrin polymers with protein located within the platelet plasma membrane at lipid rafts.1
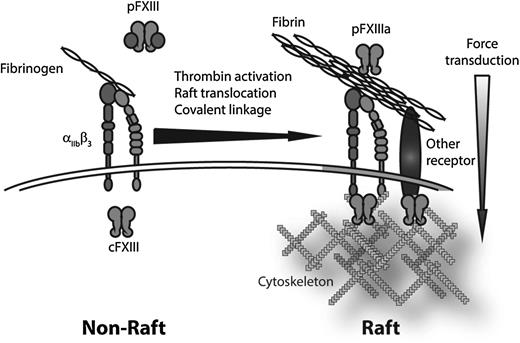
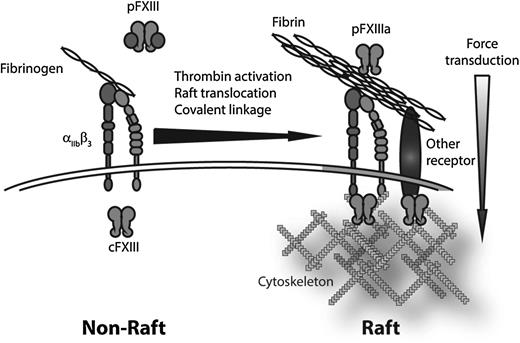
Schematic representation of translocation of fibrin to lipid rafts. On platelet activation, fibrin(ogen) binds to integrin αIIbβ3. In the presence of FXIII, the αIIbβ3-finbrin(ogen) complex translocates to SM-rich rafts. FXIIIa crosslinks fibrin fibrils to each other and covalently links fibrin to αIIbβ3 and possibly other, as yet unidentified, receptors. Concurrently, translocation of cFXIII to the lipid raft–associated actin cytoskeleton would promote crosslinking of receptor cytoplasmic domains to the cytoskeleton. Together, FXIII-dependent crosslinking would cement extracellular fibrin cables to the intracellular platelet cytosolic motors to promote clot retraction.
Schematic representation of translocation of fibrin to lipid rafts. On platelet activation, fibrin(ogen) binds to integrin αIIbβ3. In the presence of FXIII, the αIIbβ3-finbrin(ogen) complex translocates to SM-rich rafts. FXIIIa crosslinks fibrin fibrils to each other and covalently links fibrin to αIIbβ3 and possibly other, as yet unidentified, receptors. Concurrently, translocation of cFXIII to the lipid raft–associated actin cytoskeleton would promote crosslinking of receptor cytoplasmic domains to the cytoskeleton. Together, FXIII-dependent crosslinking would cement extracellular fibrin cables to the intracellular platelet cytosolic motors to promote clot retraction.
The formation of hemostatic clots begins with deposition of platelets in the area of vascular injury. The activated platelets adhere to each other, with fibrinogen and von Willebrand factor serving as intermediaries, and provide a surface for the assembly of coagulation complexes that produce thrombin, which catalyzes the formation of fibrin polymers from fibrinogen. After the clot forms, it retracts to consolidate volume and minimize leakage, a process during which platelets pull on the fibrin polymers.
The thrombin generated on the platelet surface also converts plasma FXIII to its active form FXIIIa. FXIII is a member of the transglutaminase family of enzymes, which crosslink proteins by catalyzing the formation of isopeptide bonds between glutamine and lysine residues.2 FXIII has two forms: a plasma form (pFXIII) that is a tetramer of two carrier B-subunits and two catalytic A-subunits3 and an intracellular form (cFXIII) that consists of two catalytic A-subunits. pFXIII circulates as a zymogen that must be activated by thrombin; cFXIII is constitutively active.2 FXIIIa crosslinks not only fibrin, but also crosslinks α2 antiplasmin to fibrin (which inhibits clot lysis) and fibronectin to fibrin. FXIII also has other substrates, which include factor V, Plasminogen activator inhibitor-2, collagen, and the intracellular proteins filamin, actin, and myosin.
The importance of FXIII is underscored by the clinical signs and symptoms of its deficiency. Clots formed in the absence of FXIII are unstable and easier to lyse by the fibrinolytic system, the consequence being delayed bleeding after injury or surgery. Affected individuals also experience delayed wound healing, possibly due to the inability of clots to retract properly. In affected women, habitual abortion is common, most likely due to combined abrogation of fibrin/fibronectin crosslinking by FXIII and impairment of firm adhesion of the placenta to the endometrium.
Clot retraction requires both platelets and fibrin. Platelets anchor fibrin to their surface and intracellular motors act as a winch to contract the polymerized fibrin fibers. Integrin αIIbβ3 on platelets is required to retract clots, because patients with Glanzmann thrombasthenia, who lack the receptor, exhibit defective clot retraction. In mice, FXIII has also been shown to be necessary.4
Using elegant microscopy and biochemical approaches, Kasahara et al show for the first time that fibrin associates with lipid rafts on the platelet surface and that raft integrity is required for clot retraction; raft disruption with methyl-β-cyclodextrin (which removes membrane cholesterol) prevented fibrin association and clot retraction. Using raft-specific affinity labels, they demonstrated that fibrin associates with a subset of lipid rafts that are rich in sphingomyelin (SM) (as opposed to the cholesterol-rich raft population). Platelets from mice lacking either of the enzymes that synthesize sphingomyelin retracted clots poorly.
Previous studies have implicated both pFXIII and cFXIII in fibrin association with the platelet surface on thrombin receptor activation.5 Here, Kasahara et al demonstrate that FXIII is required for translocation of fibrin to SM-rich rafts and that this fibrin is a substrate for FXIII. The translocation required the γ-chain of fibrin: a recombinant γ-chain fusion protein bound to rafts in a FXIII-dependent manner in thrombin-activated platelets. Covalent crosslinking was required for this association because mutating the FXIII crosslinking sites prevented association of the γ-chain fusion protein with rafts, as did the transglutaminase inhibitor cystamine.
These results point to a novel 2-step mechanism whereby fibrin(ogen) binds to integrin αIIbβ3, translocates to SM-rich lipid rafts in a FXIII-dependent manner, and covalently associates with an as yet unidentified receptor. Considered in light of the data indicating that cFXIII translocates to the cytoskeleton of activated platelets6 (which in activated platelets attaches to lipid rafts7 ), a mechanism emerges whereby, within SM-rich rafts, FXIII crosslinks cytoskeletal and motor elements to promote force transduction from the platelet interior (see figure). The raft-associated fibrin is crosslinked to an unknown receptor (one candidate being αvβ3), which itself might be crosslinked to the cytoskeleton on the cytoplasmic side to allow the strongest possible linkage between the cytosolic motors and the extracellular cables they tug on to retract the clot. Lack of FXIII would not only be expected to increase fibrin clot lysis, it could also impair that ability of the fibrin to attach to the underlying platelets. These findings also suggest that changes in lipid raft structure or organization could alter clot retraction and impair wound healing.
As with many thought-provoking papers, the current paper raises many new questions and opens new areas of research. These include the following. What is the receptor for fibrin within the SM-rich rafts? Is this mechanism specific for thrombin-activated platelets or is it applicable to platelet activation by other agonists? Is there a role for cFXIII-mediated crosslinking of intracellular proteins within lipid rafts? What effect does this crosslinking have on the platelets' ability to generate contractile forces? Answers to these and other questions will provide further insight into the clinical signs and symptoms of FXIII deficiency.
Conflict-of-interest disclosure: The authors declare no competing financial interests.