Key Points
BDR is an active regimen and induces long-lasting responses in patients with newly diagnosed WM.
Induction with single-agent bortezomib may be effective in preventing complications of hyperviscosity or rituximab-induced IgM flare.
Abstract
In this phase 2 multicenter trial, we evaluated the activity of bortezomib, dexamethasone, and rituximab (BDR) combination in previously untreated symptomatic patients with Waldenström macroglobulinemia (WM). To prevent immunoglobulin M (IgM) “flare,” single agent bortezomib (1.3 mg/m2 IV days 1, 4, 8, and 11; 21-day cycle), was followed by weekly IV bortezomib (1.6 mg/m2 days 1, 8, 15, and 22) every 35 days for 4 additional cycles, followed by IV dexamethasone (40 mg) and IV rituximab (375 mg/m2) in cycles 2 and 5. Fifty-nine patients were treated; 45.5% and 40% were high and intermediate risk per the International Prognostic Scoring System for WM. On intent to treat, 85% responded (3% complete response, 7% very good partial response, 58% partial response [PR]). In 11% of patients, an increase of IgM ≥25% was observed after rituximab; no patient required plasmapheresis. After a minimum follow-up of 32 months, median progression-free survival was 42 months, 3-year duration of response for patients with ≥PR was 70%, and 3-year survival was 81%. Peripheral neuropathy occurred in 46% (grade ≥3 in 7%); only 8% discontinued bortezomib due to neuropathy. BDR is rapidly acting, well tolerated, and nonmyelotoxic, inducing durable responses in previously untreated WM. This trial was registered at www.clinicaltrials.gov as #NCT00981708.
Introduction
Waldenström macroglobulinemia (WM) is a rare B-cell low-grade lymphoma, which is characterized by infiltration of the bone marrow (BM) by lymphoplasmacytic cells which produce monoclonal immunoglobulin M (IgM). Symptomatic disease is a result of tumor infiltration and/or properties and amount of the monoclonal IgM.1,2 Alkylating agents and nucleoside analogs were the backbone of therapy for WM for several decades. Rituximab has been widely used for the treatment of WM and has minimal toxicity, but as a monotherapy it is associated with modest response rates.3-6 Treatment with rituximab is also associated with a transient increase of serum IgM (“IgM flare”) in 30% to 80% of patients3,7,8 which may exacerbate complications associated with the high levels of paraprotein such as hyperviscosity syndrome.7,8 Combinations of rituximab with chemotherapy (such as the dexamethasone, rituximab, and cyclophosphamide [DRC] regimen) are associated with better response rates than rituximab alone, however, complete responses are infrequent and median time to response is ∼4 months.9 Combinations with more intensive chemotherapy (such as rituximab, cyclophosphamide doxorubicin, vincristine, and prednisone) or nucleoside analogs (such as fludarabine and rituximab or fludarabine, cyclophosphamide, and rituximab or cladribine-R) may be associated with higher response rates but at the expense of higher toxicity.10,11
Novel agents offer an opportunity to improve therapy of WM, by targeting pathways of critical importance for the survival of lymphoplasmacytic cells. Bortezomib is a proteasome inhibitor that targets multiple pathways through inhibition of protein homeostasis within cancer cells, especially plasma cells and lymphoplasmacytic cells.12-14 Bortezomib has shown in vitro activity against WM cells12,13 and significant clinical activity.15-17 In addition, bortezomib monotherapy can induce rapid reduction of IgM levels.15,16 Furthermore, synergistic activity of bortezomib with rituximab and/or steroids, has been demonstrated in vitro.18,19
Thus, in 2006, we designed a large phase 2 study to evaluate the activity of the combination of bortezomib, dexamethasone, and rituximab (BDR) in previously untreated patients with symptomatic WM.
Study design and treatment
This was a prospective, phase 2, multicenter study which enrolled patients from 10 European sites, within the context of the European Myeloma Network (EMN), after approval by national and institutional authorities. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients signed an informed consent before any procedure related to the study. Bortezomib was provided by Janssen-Cilag and rituximab was provided by Hoffman-La Roche.
The primary objective of the study was the determination of response rate (combined complete response [CR] + partial response [PR] + minimal response [MR]) in patients with previously untreated WM. Secondary objectives were the determination of time to progression following treatment with BDR and the assessment of the safety and tolerability of BDR.
The study included patients with the diagnosis of WM, based on consensus criteria,20 with symptomatic disease requiring therapy20,21 who had not received prior therapy. Symptomatic disease was defined by the presence of at least 1 of the following: B symptoms, hyperviscosity, lymphadenopathy either symptomatic or bulky (>5 cm maximum diameter), symptomatic hepatomegaly or splenomegaly, organ or tissue infiltration, peripheral neuropathy related to WM, light chain (AL) amyloidosis related to WM, nephropathy related to WM, symptomatic cryoglobulinemia, cold agglutinin anemia, immune hemolytic anemia and/or thrombocytopenia, hemoglobin <10 g/dL, platelets <100 × 109.
Eligible patients had platelets >50 × 109/L, absolute neutrophil count >750 × 106/L, Karnofsky performance status >60%, aspartate aminotransferase and alanine aminotransferase <3 upper limit of normal, total bilirubin <2 upper limit of normal, and creatinine clearance >30 mL per minute. Exclusion criteria were prior systemic therapy (plasmapheresis was allowed but per protocol no prophylactic plasmapheresis was mandated), neuropathy with or without pain ≥grade 2, poorly controlled cardiovascular disorders, mental illnesses, cardiac amyloidosis, and pregnant or breastfeeding women.
BDR regimen
A total of 5 cycles of therapy were planned. Treatment consisted of intravenous (IV) bortezomib at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 for the first 21-day cycle. On cycles 2 to 5, bortezomib was administered IV weekly at a dose of 1.6 mg/m2 on days 1, 8, 15, and 22 in four 35-day consecutive cycles. On cycles 2 and 5, IV dexamethasone 40 mg and IV rituximab at a dose of 375 mg/m2 were given on days 1, 8, 15, and 22 (total of 8 infusions of rituximab). Premedication with 1000 mg of acetaminophen and 50 mg of IV diphenhydramine were given prior to rituximab infusion. In all patients, prophylactic valacyclovir or acyclovir for herpes zoster was mandated. Bortezomib could be reduced from 1.6 to 1.3 and 0.8 mg/m2 for toxicity.
Efficacy and safety assessments
Levels of monoclonal IgM were evaluated after each cycle. Computed tomography (CT) scans of chest, abdomen, and pelvis within 3 months of study enrollment were assessed and repeated after completion of BDR if no monoclonal gammopathy was detected and if screening CT scans demonstrated evidence of disease or if progression of disease was suspected. Patients were followed every 3 months for 2 years after the last dose of study treatment, and every 6 months thereafter. When progressive disease was confirmed, patients were removed from the study. All patients that received at least 1 dose of treatment were eligible for evaluation of response and toxicity. Response was assessed on an intention-to-treat basis. The evaluation of response was performed according to the recommendations of the Third International Workshop for Waldenström’s Macroglobulinemia22 but data to evaluate cases according to the new criteria23 were also available (supplemental Table 1, available on the Blood Web site). Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (NCI CTC version 3.0).
Statistical analysis
According to Simon’s 2-stage optimal design, sample size calculation was based on the assumption that the expected overall response rate would be ≥70% and the minimum acceptable response rate 50%, for a probability of accepting a treatment with a real response rate <20% of 5% and rejecting a treatment with a response rate >40% of 10%. Progression-free survival (PFS) was measured from the date of inclusion in the study until the date of progression or death by any cause. Overall survival was calculated from the date of inclusion in the study until the date of death or date of last contact. Time to next treatment was calculated from the date of first BDR dose until the date of initiation of subsequent therapy. Duration of response was defined by the date of first documentation of response until the date of first evidence of relapse/progression or death. Survival curves were plotted with the method of Kaplan-Meier. Cause of death was defined as a result of the disease or treatment complications, or as death unrelated to WM. Unrelated deaths and WM-related deaths, progression, or reintroduction of next therapy were considered as competing events for overall survival (OS), PFS, or time to next treatment, respectively.24,25 Analysis was performed using SPSS version 20 and R software.
Results
From March 2007 until June 2010, 60 patients were enrolled in 5 European countries (Greece, Spain, Italy, France, and The Netherlands). One patient did not receive any therapy and was not included in the analysis. Patients’ characteristics are listed in Table 1. Median age was 70 years (range, 40-83 years) and median level of serum monoclonal protein was 3.86 g/dL (range, 0.17-9.9 g/dL). Most patients had advanced disease and adverse prognostic factors such as advanced age (61% were >65 years), anemia (hemoglobin <11.5 g/dL in 82%), and elevated β2-microglobulin (≥3 mg/dL in 64%). Almost half (45%) were rated as high risk, 40% were at intermediate risk, and only 15% at low risk according to International Prognostic Scoring System for Waldenström macroglobulinemia (ISSWM).26 Primary reasons for initiating treatment were cytopenias (44%), hyperviscosity (20%), B symptoms (19%), and lymphadenopathy (9%). No patient received prophylactic plasmapheresis before initiation of BDR. Per protocol patients with preexisting neuropathy grade ≥2 were not enrolled in the study. However, 7 (12%) patients had mild (grade 1) preexisting neuropathy, 1 (2%) had cryoglobulinemia, and 4 (7%) had AL amyloidosis.
Response
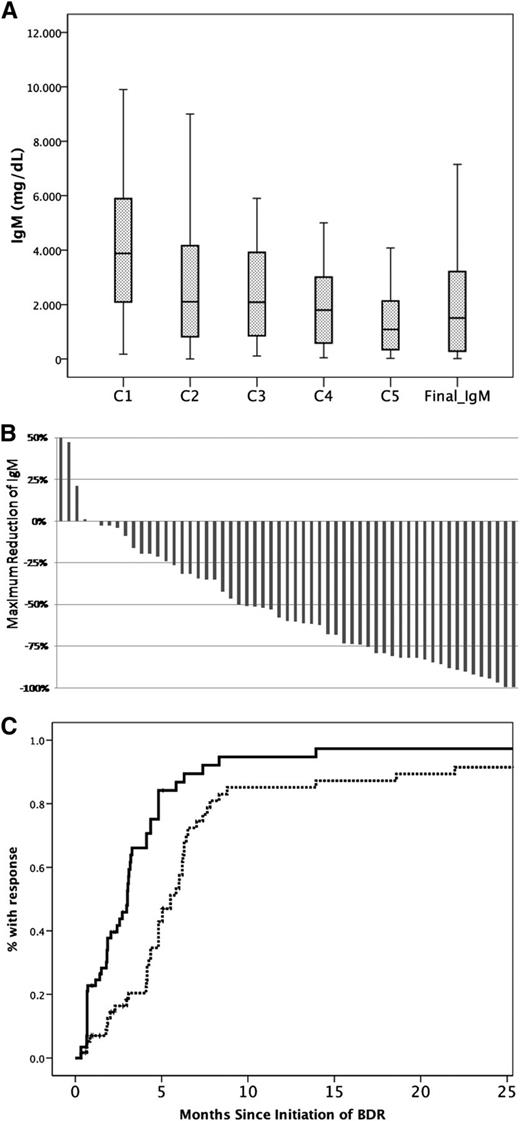
Thirty-eight (64%) patients completed the planned 5 courses; 3 (5%) received 4 cycles, 7 (12%) received 3 cycles, 3 (5%) received 2 cycles, and 8 (14%) patients received only the first cycle of single-agent bortezomib. The overall response rate was 85% (95% CI, 73%-92%): 2 patients achieved CR (3%), 38 (65%) PR, and 10 (17%) MR; a major response (≥PR) was achieved by 68% (95% CI, 55%-78%). Four (7%) patients had ≥90% reduction of IgM but had positive immunofixation. Thus, according to the recently proposed criteria,23 the very good partial response (VGPR) rate was 7%. Three (5%) patients had stable disease whereas 6 (10%) were rated as progressive disease (Figure 1B; Table 2). Among patients with evaluable lymphadenopathy, 35% had complete resolution and 40% at least partial resolution of their lymphadenopathy. Splenomegaly resolved completely in 55% and partially in 33% of evaluable patients. Overall, 63% of patients with organomegaly (lymphadenopathy, splenomegaly, or both) achieved a response. Among patients who responded, CR, VGPR, PR, and MR rates were 6%, 9%, 71%, and 14%, respectively, for those who received all 5 cycles, whereas for responding patients who received <5 cycles of the VGPR, PR, and MR rates were 7%, 57%, and 36%, respectively (no CRs). Median time to first response (≥MR) was 3 months and median time to best response was 5 months; however, 4 (8%) of the responders achieved their best response >6 months after completion of therapy (Figure 1C). No patient required plasmapheresis during therapy with BDR, despite the fact that 44% of the patients had IgM levels ≥4000 mg/dL. Among patients (N = 28) who had a posttreatment BM biopsy, median BM infiltration reduced from 62% to 14% (median reduction, 57%) and the respective median IgM reduced from 3835 mg/dL to 1680 mg/dL (median reduction, 60%); however, the correlation was not very strong (R2: 0.124, P = .1).
Response of serum IgM to treatment with BDR. Reduction of IgM levels (A) after each cycle of BDR and at final assessment 3 months postcompletion of BDR. (B) Maximum decrease of the IgM in individual patients. (C) Time to first response (solid line) and time to best response (dotted line).
Response of serum IgM to treatment with BDR. Reduction of IgM levels (A) after each cycle of BDR and at final assessment 3 months postcompletion of BDR. (B) Maximum decrease of the IgM in individual patients. (C) Time to first response (solid line) and time to best response (dotted line).
IgM levels after bortezomib monotherapy and “IgM flare”: after the first cycle of single-agent bortezomib, median reduction of IgM was 18% (range, −78% to +12%; 34% of patients had ≥25% reduction and 8% had ≥50% reduction) (Figure 1A). In 11% of patients, a ≥25% increase of IgM was observed after the second cycle of therapy (which included rituximab); the median IgM rise in these patients was 60% (range, 39%-219%) and the median absolute increase of IgM was 1614 mg/dL (range, 580-4610 mg/dL). This rise of IgM was followed by a subsequent drop of IgM levels after cycle 3, which included bortezomib only. No patient required plasmapheresis for symptomatic hyperviscosity or other complications of the transient IgM increase during therapy with BDR. Among the patients who experienced the IgM rise, 5 (50%) had a PR as their best response, 2 (20%) had a MR, 1 had stable disease, and 2 (20%) were rated as progressive disease. A mild, less pronounced, rise of IgM (median IgM rise, 31% and median absolute IgM rise, 290 mg/dL; range, 170-3440 mg/dL) was also observed in 20% of evaluable patients after the second block of rituximab (cycle 5); no intervention was required.
Time to progression, further therapy, and survival
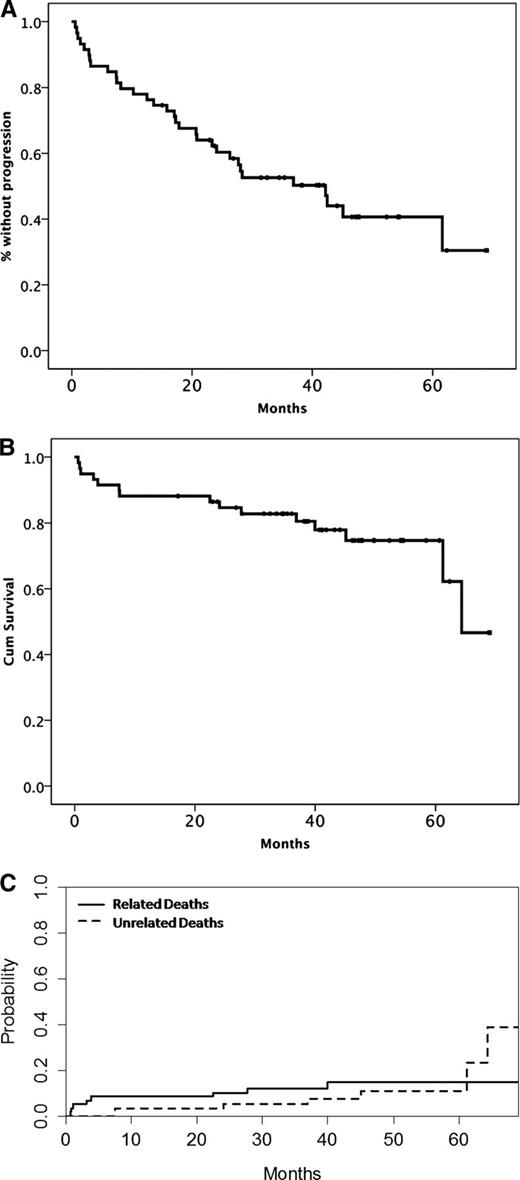
Minimal follow-up after the last patient’s entry in the study was 32 months and median follow-up for all patients was 42 months. Until the date of data cutoff (February 1, 2013), 32 (54%) patients experienced disease progression or died (27 had disease progression and 5 died without evidence of disease progression). The estimated median PFS was 42 months, the 3-year cumulative rate of progression was 41%, and the 3-year unrelated death rate without progression is 9%. For patients who achieved at least PR, the 3-year progression rate was 22.5% and the unrelated death rate was 8% (Figure 2A; supplemental Figure 1). Accounting for the competing risk of unrelated death, the 3-year risk of progression for patients with a VGPR or better, PR, and MR was 0%, 41%, and 70%, respectively (P = .02).The 3-year progression probability was 12.5%, 45%, and 46% for low-, intermediate-, and high-risk patients per ISSWM, respectively (P = .517), and the respective risk of unrelated death was 0%, 4.5%, and 8% (P = .648). PFS for responding patients who received <5 cycles was shorter than those who completed 5 courses (28 vs 62 months, P = .003).
Time-to-event curves after therapy with BDR. (A) PFS, (B) OS, and (C) cumulative incidence of related and unrelated deaths.
Time-to-event curves after therapy with BDR. (A) PFS, (B) OS, and (C) cumulative incidence of related and unrelated deaths.
Nineteen patients received further treatment on progression and 84% of them achieved ≥MR. Most (89%) received rituximab-based therapy (DRC [N = 6], rituximab-cyclophosphamide, vincristine, and prednisone [N = 1], rituximab alone [N = 1], rituximab, cyclophosphamide doxorubicin, vincristine, and prednisone [N = 5], fludarabine, cyclophosphamide, and rituximab [N = 2], bendamustine-rituximab [N = 1]); 1 patient received fludarabine and cyclophosphamide and 1 alemtuzumab (no information was available about the type of therapy in 1 patient). The 3-year rate of subsequent therapy was 28% and 3-year unrelated death rate without subsequent treatment was 7% (supplemental Figure 1). Fifteen patients have died; 7 patients died due to causes unrelated to WM or therapy (5 of cardiovascular diseases, 1 died of pneumonia, and 1 due to complications after surgery for lung cancer). The 3-year survival rate is 82%, and the 3-year cumulative incidence of WM-related deaths was 12%, and of unrelated deaths was 5%.
There was no significant association of any of the baseline features of the disease (cytopenias, β2-microglobulin, IgM levels, BM infiltration, splenomegaly, lymphadenopathy) or of the patients’ characteristics (age, gender, performance status) or ISSWM with the probability of response (≥MR or ≥PR). Per ISSWM, the 3-year progression rate was 30%, 46%, and 43% for the low-, intermediate-, and high-risk groups, respectively (P = .8). Serum albumin <3.5 g/dL was associated with an increased risk of death due to WM (3-year risk of death of 22% vs 0% for those with serum albumin ≥3.5 g/dL, P = .03). Risk of death for different ISSWM risk groups was not significantly different, perhaps due to the small number of events.
Toxicity
Main reasons for discontinuation of BDR were toxicity in 16 (27%) patients and disease progression (or death) in 5 patients. Toxicities during BDR are depicted in Table 3. Hematologic toxicity included mainly neutropenia (grade ≥3 in 15%) and thrombocytopenia (grade ≥3 in 5%). Peripheral neuropathy of any grade was recorded in 46% (grade 2 in 17% and grade ≥3 in 7%); neuropathic pain was recorded in 20% but was grade 3 in only 1 patient. In 22 (37%) patients, the dose of bortezomib was reduced by at least 1 dose level due to neuropathy but only 5 (8%) patients discontinued bortezomib due to neuropathy. Among patients with preexisting neuropathy, cryoglobulinemia or AL amyloidosis, the rates and severity of neurotoxicity were similar to that of patients without preexisting neuropathy and most of them completed 5 courses of BDR. Infections were common and usually mild; only 1 patient developed neutropenic fever. One patient developed herpes zoster; after he discontinued prophylaxis with valacyclovir, he received full-dose valacyclovir and continued therapy with BDR and prophylaxis. One patient died due to nonneutropenic septic shock. Three (5%) patients experienced pulmonary toxicity which was attributed to bortezomib, consisting of dyspnea, decrease of arterial pO2, and diffuse pulmonary infiltrates on chest CT scan. Pulmonary toxicity in the 3 patients developed in cycle 1, cycle 4, and cycle 5, respectively, and was reversible after administration of corticosteroids. Two of the 3 patients continued treatment as per protocol and completed 5 cycles of BDR.
Discussion
This is the largest phase 2 trial of a bortezomib-based regimen in WM and with the longest follow-up. In previously untreated patients, BDR showed excellent activity, with manageable toxicity and reduced incidence and severity of bortezomib-related neurotoxicity. Importantly, the responses were durable, despite the lack of maintenance. Furthermore, this trial established a European collaboration network for the conduction of clinical studies in a rare disease such as WM.
The current study was designed on the basis of clinical and preclinical data which indicated potential additive/synergistic activity for the combination of bortezomib and rituximab.18,19 Furthermore, the design of the BDR regimen was based on the particular characteristics of WM: besides the tumor load in the BM, we also considered the effects of the circulating monoclonal IgM. Accordingly, BDR was designed not as a “lymphoma-like” but as a “WM-specific” therapy. To exploit the rapid activity of bortezomib, in terms of IgM reduction, an initial cycle of bortezomib was given before initiation of rituximab, to reduce IgM levels and subsequently the frequency and severity of rituximab-associated “IgM flare.” Additionally, we adopted a weekly schedule of administration to reduce bortezomib-related neurotoxicity.
The major response rate (CR + VGPR + PR) was 68% (85% including ≥MR), which is higher than any of the drugs alone (≥PR in 30% to 50%3-7,15-17,27 ), indicating clinical synergism between bortezomib and rituximab. The median time to first response was 3 months, and compares favorably to rituximab alone (median time to response ≥6 months3,4,6,7 ). The median time to best response was 5 months, but some patients achieved their best response several months after completion of BDR despite the fact that there was no maintenance. The response rates in our study are similar to those reported by Ghobrial et al in a smaller study of bortezomib-rituximab which also did not include maintenance (≥MR in 88%, 8% CR + near CR and 58% PR).28 In the BDR study by Treon et al,29 responses were higher (≥PR 83%, CR 13%, near CR 9%, VGPR 13%) perhaps due to the use of maintenance with additional BDR cycles.
The duration of response after BDR is also favorable. In contrast to previous studies,28,29 our study has sufficient follow-up to assess PFS, with a long median of 42 months, although there was no maintenance. Notably, 85% of our patients were rated intermediate or high risk per ISSWM. Of note, the 3-year OS is 82% and the 3-year cumulative incidence of WM-related deaths and unrelated deaths is 12% and 5%, respectively. The quality of response to primary therapy may be associated with survival in lymphoproliferative disorders. In patients with WM, the depth of the response may be associated with longer PFS,30 which is what we also observed in our study, however, several years of follow-up are needed to evaluate the effect of the therapy and the quality of response on survival. Future clinical studies should aim at the development of regimens with higher CR rates and reasonable toxicity.
Induction with single-agent bortezomib was an effective strategy to manage complications associated with high IgM levels and reduced the need for plasmapheresis and the frequency and severity of rituximab-associated “IgM flare”: only 11% of patients had an IgM rise after rituximab and there was no need for plasmapheresis although 44% of patients had IgM ≥4000 mg/dL before initiation of therapy. In comparison, “IgM flare” was observed in 54% of patients treated with single-agent rituximab,3,7 in 32% of patients after DRC,9 and in 31% of patients treated with rituximab-bortezomib.28 In the BDR study by Treon et al, prophylactic plasmapheresis was performed in 26% of patients but 9% had an “IgM flare” requiring plasmapheresis.29
Toxicity is a major concern for patients with WM, especially insofar as most of them are elderly. BDR had limited myelotoxicity, thus, it may be an attractive option for patients who present with cytopenias or for patients who are candidates for autologous stem-cell transplant because none of the drugs is stem cell toxic. The weekly administration of bortezomib was well tolerated, with low rates of clinically significant neuropathy and, importantly, only 8% of patients discontinued bortezomib due to neuropathy, comparing favorably to previously published studies which used a twice-per-week schedule (discontinuation rates of 25%-61%16,17,29 ), and in accordance with data from weekly administration of bortezomib with rituximab.28 However, neurotoxicity must be evaluated with caution and should be considered when physicians decide the most appropriate therapy for patients with WM because in a disease with prolonged survival such as WM, neuropathy may seriously affect quality of life. Fortunately, neuropathy was completely reversible in most of our patients. Furthermore, with the use of subcutaneous bortezomib, the problem of peripheral neuropathy is likely to become less important.31 In addition, the development of a novel generation of proteasome inhibitors that seem to be less or not neurotoxic favors the continuous study of the therapeutic impact of proteasome inhibitors in WM. Pulmonary toxicity is an uncommon complication of bortezomib.32-36 In our study, it occurred in 5% of patients, but improved rapidly with steroids and standard supportive measures, and actually 2 of 3 patients continued and completed BDR. Physicians who treat patients with bortezomib must be aware of this uncommon complication and evaluate those patients who present with pulmonary symptoms accordingly and initiate steroids if indicated.
Our results justify BDR as an alkylator-free, primary treatment option for patients with WM. The updated results from the phase 2 study of primary therapy with DRC indicated a median PFS of 35 months and 5-year OS of 62%. BDR is associated with similar response rates and a PFS of 42 months but further follow-up is needed for the assessment of survival. Both regimens are active, but with different toxicity profiles and both may be considered as primary therapy in different indications. BDR may be preferable for patients with high levels of IgM, symptoms of hyperviscosity, or severe cytopenias whereas DRC may be preferred for patients with lower levels of IgM, less pronounced cytopenias, IgM-related neuropathy or patients who do not wish to return to the hospital frequently for bortezomib injections.
In summary, primary therapy with BDR is safe and effective, associated with maintained responses and excellent PFS in previously untreated patients with WM. Induction with single-agent bortezomib effectively reduces IgM levels and may reduce rituximab-associated “IgM flare” and the need for plasmapheresis, whereas weekly administration of bortezomib reduces the risk of neurotoxicity.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A.D. designed the study, analyzed data, and wrote the manuscript; R.G.-S. analyzed and collected data and critically reviewed the manuscript; M.G. collected and analyzed data and critically reviewed the manuscript; P.M. analyzed and collected data, performed statistical analysis, and critically reviewed the manuscript; M.-C.K., E.M., Z.K., X.L., G.P., A.T., D.G., and G.M. analyzed and collected data and critically reviewed the manuscript; E.K. analyzed and collected data, performed statistical analysis, and wrote the manuscript; and P.S. designed the study, analyzed and collected data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: M.A.D. received honoraria from Janssen and Celgene and research support from Janssen, Celgene, Millennium-Takeda, and Onyx. R.G.-S. received research support from Novartis SA and honoraria from Millennium-Takeda, Janssen-Cilag, and Pharmacyclics. G.P. received honoraria from Millennium-Takeda and Pfizer and research support from Pfizer. G.M. received honoraria from Millennium-Takeda, Pfizer, and Onyx. P.S. received honoraria from Janssen and Celgene and research support from Janssen, Celgene, Millennium-Takeda, and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Meletios A. Dimopoulos, Department of Clinical Therapeutics, National and Kapodistrian University of Athens School of Medicine, 80 Vas. Sofias Ave, 115 28 Athens, Greece; e-mail: mdimop@med.uoa.gr.