Key Points
Soluble blood-borne antigens are crosspresented in the BM, triggering T-cell arrest, clustering, and in situ proliferation.
In the BM, not only DCs but also other mononuclear phagocytes participate in crosspresentation.
Abstract
The bone marrow (BM) hosts memory lymphocytes and supports secondary immune responses against blood-borne antigens, but it is unsettled whether primary responses occur there and which cells present the antigen. We used 2-photon microscopy in the BM of live mice to study these questions. Naïve CD8+ T cells crawled rapidly at steady state but arrested immediately upon sensing antigenic peptides. Following infusion of soluble protein, various cell types were imaged ingesting the antigen, while antigen-specific T cells decelerated, clustered, upregulated CD69, and were observed dividing in situ to yield effector cells. Unlike in the spleen, T-cell responses persisted when BM-resident dendritic cells (DCs) were ablated but failed when all phagocytic cells were depleted. Potential antigen-presenting cells included monocytes and macrophages but not B cells. Collectively, our results suggest that the BM supports crosspresentation of blood-borne antigens similar to the spleen; uniquely, alongside DCs, other myeloid cells participate in crosspresentation.
Introduction
The bone marrow (BM) has long been considered the primary organ of hematopoiesis. More recently, the BM was shown to also support long-term survival of certain terminally differentiated lymphocytes,1 attracting long-lived plasma cells2 and hosting mature recirculating B cells.3
Emerging evidence indicates that memory CD4+ T cells also reside in the BM and can be activated there.4,5 Moreover, in both humans6,7 and mice,7,8 memory CD8+ T cells preferentially accumulate in the BM, where they display an activated profile9 and proliferate extensively.8 Indeed, activated CD8+ T cells from the BM were more cytotoxic toward tumors than their blood counterparts.6 These findings suggest that BM-resident T cells are functionally superior but do not indicate which cellular interactions contribute to their advantage.
Apart from memory T lymphocytes, the BM also contains naïve T cells, albeit in smaller numbers.7 Usually disregarded, these cells may still participate in primary immune responses to blood-borne antigens.10 The cellular interactions enabling presentation of blood-borne antigens in the BM and the local generation of bona fide T-cell responses have not been fully studied, let alone imaged.
Dendritic cells (DCs) are antigen-presenting cells (APCs) considered essential for activating primary T-cell responses.11 They outperform other APCs in priming naïve T cells,12 and their specific ablation abolishes crosspriming of CD8+ T cells against intracellular pathogens.13 Correspondingly, Cavanagh et al14 used 2-photon microscopy (2PM) to show that transferred antigen-pulsed DCs can migrate to the BM and form antigen-dependent contacts with central memory T cells. We previously showed that BM-resident DCs occupy perivascular niches and provide survival signals to mature B cells.15 However, we have not established the origin of these DCs or their role in initiating immune responses.
Here, we investigated whether the BM supports crosspresentation of blood-borne antigens to naïve CD8+ T cells. We followed each step of this process using 2PM and cytometric and functional assays. Finally, we studied the identity of the APCs that take up the antigen and crosspresent it to the T cells.
Our results indicate that, in the BM, blood-borne antigens are crosspresented to naïve CD8+ T cells, promoting local T cell arrest, activation, proliferation, and effector cell generation. Unlike the spleen, which relies on DCs alone for crosspresentation, the BM also employs monocytes/macrophages.
Material and methods
Mice
We used C57BL/6 (either CD45.2 or CD45.1); CD11c-DTR transgenic (B6.FVB-Tg Itgax-DTR/EGFP 57Lan/J) mice transgenic for enhanced green fluorescent protein (EGFP) fused to human diphtheria toxin receptor (DTR) under the CD11c promoter13 ; Cx3cr1gfp/+ mice (B6.129P-Cx3cr1tm1Litt/J)16 as well as CD11c-DTRtg×Cx3cr1gfp/+ crosses; OT-I T-cell receptor (TCR) transgenic mice17 ; β-actin–driven cyan fluorescent protein (CFP)18 mice; H2-Kb-deficient mice19 ; interleukin-15 (IL-15)–deficient mice, which lack natural killer cells20 ; and JH−/− mice, which lack B cells.21 The following chimeric mice were generated as reported previously22 : CD11c-DTRtg into wild-type C57BL/6, CD11c-DTRtg× Cx3cr1gfp/+ into wild-type, CD11c-DTRtg into H2-Kb−/−, CD11c-DTRtg into IL-15−/−, and H2-Kb−/− into IL-15−/−.
Mice were 8 to 12 weeks old and were maintained in specific pathogen-free conditions as approved by the Weizmann Institute Animal Care Committee.
Adoptive cell transfer
Syngeneic B cells were purified from spleens and lymph nodes (LNs) of β-actin–driven CFP mice by depletion with immunomagnetic anti-CD43 beads (Miltenyi Biotec). Polyclonal and OT-I CD8+ T cells were positively selected from spleens and LNs with anti-CD8α beads (Miltenyi Biotec). For intravital imaging, we used endogenously fluorescent cells or labeled them with 5 µM CMTMR (CellTracker orange, Invitrogen) for 20 minutes at 37°C in serum-free conditions. Then, 5 to 8 × 107 cells were injected intravenously. Typically, 2 types of cells were coinjected at a 1:1 ratio to allow for internal comparisons.
To assess in vivo T-cell proliferation, OT-I cells were labeled with 5 µM carboxyfluorescein succinimidyl ester (CFSE) or CellTrace violet (Invitrogen) according to the manufacturer’s instructions and 1 × 107 were injected intravenously. Dye dilution was quantified at 48 hours using flow cytometry.
In vivo killing
A standard CFSE-based assay was used. For further details, please see supplemental Methods.
Antigen administration
To analyze T-cell responses, mice were intravenously injected with 50 μg SIINFEKL peptide + 0.1 mg 500 kDa dextran-FITC (Sigma) or with 0.1 to 0.5 mg ovalbumin (OVA) protein (Sigma). For in vivo imaging of antigen uptake, 0.1 mg OVA–Alexa 594 (Invitrogen) or 0.5 mg OVA–Texas red (Invitrogen) was injected intravenously. When indicated, 10 nmol cytosine-guanine oligodeoxynucleotides (CpG; TIB MOLBIOL) was injected intravenously.
Cell ablation
For DC ablation, chimeric mice reconstituted with CD11c-DTRtg cells were injected intraperitoneally with 8 μg/kg diphtheria toxin (DTx; Sigma). To deplete monocytes and macrophages, mice were intravenously injected with 400 μL clodronate-encapsulating liposomes (CLL) solution prepared as described previously23 and provided by Dr. Nico van Rooijen. Clodronate was a gift of Roche Diagnostics GmbH. In both cases, repeated administration was used for long-term depletion.
Intravital 2PM of the BM
We adapted a previously described protocol.24 For further details, see supplemental Methods.
Statistics
Two-tailed t tests were used to compare 2 samples. Unless indicated otherwise, multiple comparisons were performed using 2-way analysis of variance (ANOVA) with Bonferroni correction for planned comparisons (performed within each organ against OVA-treated mice). Significance was set at P < .05. Analysis was performed using GraphPad Prism or StatView (SAS Institute) software.
For further details, please see supplemental Methods.
Results
The BM hosts naïve CD8+ T cells that crawl rapidly and respond to blood-borne antigens
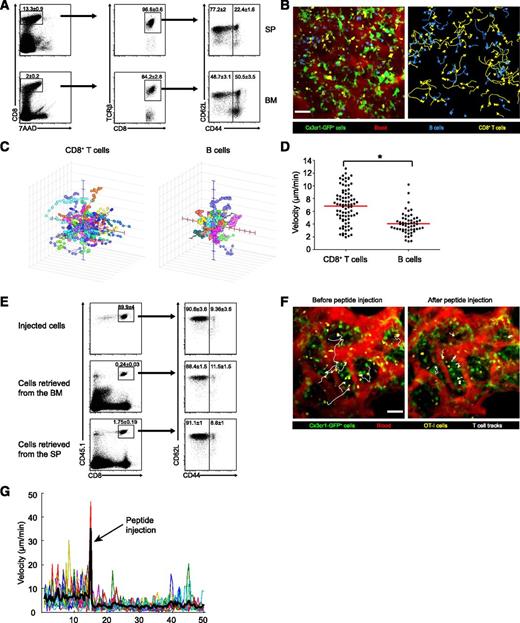
The BM is known to host plasma cells and memory T cells,2,5,7,8 but the population of naïve T cells and their ability to mount primary immune responses to blood-borne antigens in this organ has received less attention.10 We first compared the profile of CD8+ T cells in the BM and spleen. As previously reported,7 the BM was a major reservoir of memory CD44+ TCRβ+ CD8+ T cells (Figure 1A). Nonetheless, it also hosted a sizable population of naïve CD44− cells, comprising around half of the CD8+ T cells (compared with ∼77% in the spleen).
Naïve CD8+ T cells abound in the BM, crawl rapidly, and respond to blood-borne antigens. (A) Flow cytometry of CD8α+ TCRβ+ T cells in the BM and spleen of wild-type mice; dead cells were excluded by 7AAD staining. Memory T cells (CD44+) and naïve cells (CD44−) are visible. Naïve cells represent ∼77% of CD8 T cells in the spleen and ∼50% of CD8 T cells in the BM. Numbers indicate mean percentage ± standard error of the mean (SEM) from 5 mice examined in 2 separate experiments. (B) An extended-focus snapshot (left) taken by 2PM of cranial BM cavity in a live mouse. Shown are CMTMR-labeled CD8+ T cells (yellow) and CFP+ B cells (blue) transferred into Cx3cr1gfp/+ mice (where DCs, monocyte, and macrophages appear green). The BM microvasculature was visualized by intravenous injection of Quantum dots (red). As evidenced by motion tracks (right panel) of CD8+ T cells (yellow) and B cells (blue), T cells share the same niches as B cells but crawl faster. Bar represents 50 µm. (C) Three-dimensional tracks of individual CD8+ T and B cells. For clarity, tracks were assigned a common origin. (D) Average track velocity of transferred CD8+ T and B cells. Data points represent individual cells (n of T cells = 83, n of B cells = 55). *P < .0001 (2-tailed t test). Data are from 1 representative experiment out of 3. (E) Flow cytometry of transferred CD8+ OT-I T cells retrieved from the spleen and BM of CD45.2+ recipient mice. For transfer, T cells from the spleen and LNs of CD45.1+ OT-I mice were immunomagnetically enriched for CD8α expression. Injected cells (top) and cells retrieved 4 hours later from the BM and spleen (middle and bottom) were gated for CD45.1+ and CD8+ expression (left) and examined for CD62L and CD44 expression (right). The percentage of naïve cells remained constant at ∼90%. Numbers indicate mean percentage ± SEM from 5 mice pooled from 2 independent experiments. (F) Extended-focus snapshots with selected cell tracks (white) of CMTMR-labeled naïve CD8+ OT-I T cells (yellow) transferred into Cx3cr1gfp/+ mice before (left) and after (right) intravenous injection of the OVA peptide SIINFEKL. The BM microvasculature was visualized using Quantum dots (red); GFP+ cells appear green. Bar represents 50 µm. (G) Instantaneous velocities during the whole imaging session of individual OT-I cells before and after peptide injection. The motion artifact introduced by peptide injection is followed by a marked decrease in cell speed. Data are from 1 representative experiment of 2. SP, spleen.
Naïve CD8+ T cells abound in the BM, crawl rapidly, and respond to blood-borne antigens. (A) Flow cytometry of CD8α+ TCRβ+ T cells in the BM and spleen of wild-type mice; dead cells were excluded by 7AAD staining. Memory T cells (CD44+) and naïve cells (CD44−) are visible. Naïve cells represent ∼77% of CD8 T cells in the spleen and ∼50% of CD8 T cells in the BM. Numbers indicate mean percentage ± standard error of the mean (SEM) from 5 mice examined in 2 separate experiments. (B) An extended-focus snapshot (left) taken by 2PM of cranial BM cavity in a live mouse. Shown are CMTMR-labeled CD8+ T cells (yellow) and CFP+ B cells (blue) transferred into Cx3cr1gfp/+ mice (where DCs, monocyte, and macrophages appear green). The BM microvasculature was visualized by intravenous injection of Quantum dots (red). As evidenced by motion tracks (right panel) of CD8+ T cells (yellow) and B cells (blue), T cells share the same niches as B cells but crawl faster. Bar represents 50 µm. (C) Three-dimensional tracks of individual CD8+ T and B cells. For clarity, tracks were assigned a common origin. (D) Average track velocity of transferred CD8+ T and B cells. Data points represent individual cells (n of T cells = 83, n of B cells = 55). *P < .0001 (2-tailed t test). Data are from 1 representative experiment out of 3. (E) Flow cytometry of transferred CD8+ OT-I T cells retrieved from the spleen and BM of CD45.2+ recipient mice. For transfer, T cells from the spleen and LNs of CD45.1+ OT-I mice were immunomagnetically enriched for CD8α expression. Injected cells (top) and cells retrieved 4 hours later from the BM and spleen (middle and bottom) were gated for CD45.1+ and CD8+ expression (left) and examined for CD62L and CD44 expression (right). The percentage of naïve cells remained constant at ∼90%. Numbers indicate mean percentage ± SEM from 5 mice pooled from 2 independent experiments. (F) Extended-focus snapshots with selected cell tracks (white) of CMTMR-labeled naïve CD8+ OT-I T cells (yellow) transferred into Cx3cr1gfp/+ mice before (left) and after (right) intravenous injection of the OVA peptide SIINFEKL. The BM microvasculature was visualized using Quantum dots (red); GFP+ cells appear green. Bar represents 50 µm. (G) Instantaneous velocities during the whole imaging session of individual OT-I cells before and after peptide injection. The motion artifact introduced by peptide injection is followed by a marked decrease in cell speed. Data are from 1 representative experiment of 2. SP, spleen.
Naïve T cells crawl vigorously in LNs25 and can home to perivascular regions in the BM when transferred,15 but their motility in the BM has not been examined. We therefore imaged adoptively transferred, fluorescently labeled lymphocytes in the intact skull bones of Cx3cr1gfp/+ anesthetized mice and compared the motility of CD8+ T and B lymphocytes. Four hours after transfer, both T and B cells had settled in the same DC-rich areas of the BM, previously termed BM immune niches15 (Figure 1B; supplemental Video 1). Tracking the 3-dimensional positions of individual cells revealed that T cells crawled at a mean velocity of 6.8 μm/min, whereas B cells were significantly slower, crawling at 4 μm/min (Figure 1C-D), a difference previously noticed in LNs.25 Thus, naïve T cells in the BM crawl faster than B cells even though, unlike in secondary lymphoid organs, they share the same niche.15 This suggests that crawling speed may be an intrinsic quality of B cells and T cells and is not strictly dictated by the architecture or molecular composition of the follicles and T cell zones, which are absent from the BM.
To study the response of naïve CD8+ T cells to TCR ligation in the BM, we adoptively transferred TCR-transgenic OT-I T cells specific to the OVA-derived SIINFEKL peptide. Cells were positively selected using anti-CD8α and analyzed for CD3, CD8, CD11c, CD44, and CD62L expression. Of the transferred cells, 93% were CD3+ CD8+ T cells, and less than 0.5% were CD11c+ (supplemental Figure 1). About 90% of the transferred CD8+ cells were naïve, and this percentage hardly changed as T cells colonized the BM and the spleen (Figure 1E).
Upon TCR stimulation, T cells receive an immediate “stop signal” that counters their chemokinetic program and immobilizes them in contact with APCs. Initially described in vitro,26 this phenomenon has directly been observed in LNs and spleen following systemic administration of antigenic peptides.27,28 We transferred OT-I cells into Cx3cr1gfp/+ recipients and 4 hours later imaged the BM as SIINFEKL was injected. In response, T cells immediately arrested (Figure 1F-G; supplemental Video 2).
BM CD8+ T cells gradually slow down, cluster, upregulate CD69, and proliferate in response to blood-borne antigen
In the previous experiment, antigenic peptides were externally loaded on all major histocompatibility complex (MHC)-I–positive cells rather than processed by APCs and loaded intracellularly on MHC-I. To mimic a more physiological setting, we challenged the mice with intact OVA protein.
We first determined the kinetics of protein uptake using fluorescently labeled OVA. We imaged the BM immune niches of Cx3cr1gfp/+ mice while intravenously injecting soluble OVA–Alexa 594. Immediately after injection, the protein was observed in the BM sinusoids, and within 3 minutes it crossed the endothelium into the BM cavity (supplemental Video 3). An hour later, OVA had been taken up by many cell types, including CX3CR1-GFP+ cells showing DC morphology (Figure 2A middle). The following day, the pattern has changed: fluorescence could now be detected almost exclusively in GFP− cells lining the BM sinusoids (Figure 2A right)—presumably endothelial cells. To further define BM cells that took up the antigen, we employed flow cytometry 2 hours after administration of OVA–Alexa 594. Neither plasmacytoid DCs (pDCs) nor T cells captured the labeled protein. Among professional APCs, B cells and macrophages accumulated it to a significant level, while DCs were the most efficient (Figure 2B).
Several types of APCs in the BM take up soluble protein from the blood. (A) 2PM images of in vivo uptake of blood-borne antigen by BM cells of a Cx3cr1gfp/+ mouse at different time points. An hour after injection of 0.1 mg of OVA–Alexa 594 (yellow), the antigen had been taken up by many cell types, including green CX3CR1-GFP+ cells (arrows, middle) exhibiting DC morphology (insets, middle). By 24 hours, the fluorescent signal (arrows, right) persisted only in flat cells that line blood vessels (red), consistent with endothelial or perivascular cells (insets, right). Data are from 1 representative experiment of 2. Bar represents 50 µm. (B) Flow cytometric analysis of uptake of blood-borne antigen by various cells in the BM. The BM was harvested 2 hours after intravenous injection of 0.1 mg OVA–Alexa 594 and compared with the BM of noninjected mice. Representative histograms depict the OVA–Alexa 594 signal in pDCs (CD11cint mPDCA-1hi), T cells (CD3hi), B cells (CD19hi), conventional DCs (cDCs) (CD11chi MHC-II+), macrophages (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II+), Ly6Clo monocytes (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II−), and Ly6Chi monocytes (CX3CR1-GFP+, CD11c−). Data are from 1 representative experiment of 3. Except for T cells and pDCs, all examined cell types took up the antigen to some extent, with cDCs being the most efficient.
Several types of APCs in the BM take up soluble protein from the blood. (A) 2PM images of in vivo uptake of blood-borne antigen by BM cells of a Cx3cr1gfp/+ mouse at different time points. An hour after injection of 0.1 mg of OVA–Alexa 594 (yellow), the antigen had been taken up by many cell types, including green CX3CR1-GFP+ cells (arrows, middle) exhibiting DC morphology (insets, middle). By 24 hours, the fluorescent signal (arrows, right) persisted only in flat cells that line blood vessels (red), consistent with endothelial or perivascular cells (insets, right). Data are from 1 representative experiment of 2. Bar represents 50 µm. (B) Flow cytometric analysis of uptake of blood-borne antigen by various cells in the BM. The BM was harvested 2 hours after intravenous injection of 0.1 mg OVA–Alexa 594 and compared with the BM of noninjected mice. Representative histograms depict the OVA–Alexa 594 signal in pDCs (CD11cint mPDCA-1hi), T cells (CD3hi), B cells (CD19hi), conventional DCs (cDCs) (CD11chi MHC-II+), macrophages (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II+), Ly6Clo monocytes (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II−), and Ly6Chi monocytes (CX3CR1-GFP+, CD11c−). Data are from 1 representative experiment of 3. Except for T cells and pDCs, all examined cell types took up the antigen to some extent, with cDCs being the most efficient.
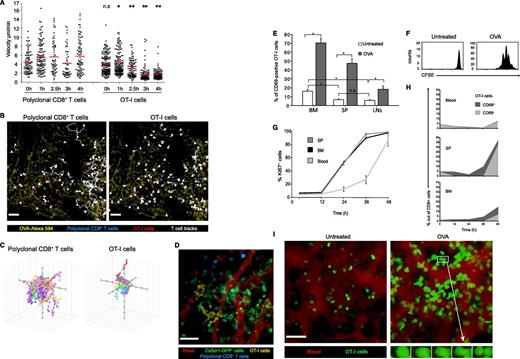
Before they can present antigens to naïve CD8+ T cells, APCs must ingest the antigen, deliver it to the cytosol, and degrade it into peptides to be loaded onto MHC-I molecules. This process, termed crosspresentation,29 is thought to be restricted to specialized APCs.30 To follow its kinetics in the BM, we first followed the deceleration of T cells, an established indicator of TCR ligation.25,27 We cotransferred wild-type mice with OT-I cells and polyclonal CD8+ T cells (as an internal control). Four hours later, mice were challenged with 0.1 mg OVA protein, and their BM was repeatedly imaged for 5 hours. In the first 2 hours, polyclonal T cells and OT-I cells crawled at similar speeds. During the next 2 hours, OT-I, but not polyclonal cells, gradually decelerated (Figure 3A-C; supplemental Video 4). As previously observed in the spleen,27 deceleration in response to intact antigen was gradual, unlike the immediate arrest after peptide administration (Figure 1F-G). These results indicate that the processing and crosspresentation of intact proteins by BM APCs is complete within 2 to 3 hours of antigen administration.
Naïve CD8+ T cells are efficiently activated in the BM upon antigenic challenge. (A) Average velocities of transferred polyclonal CFP+ CD8+ T cells and GFP+ OT-I cells, collected from the spleen and LNs, in the presence of antigenic protein. During BM imaging, the mice were challenged with 0.1 mg OVA–Alexa 594 and were continuously imaged for 5 hours. Cell speed gradually decreased in an antigen-specific manner. Data points represent individual cells taken from 1 representative experiment out of 3. *P < .05; **P < .001 (based on a comparison between polyclonal and OT-I T cells within the same time point). Multiple comparisons analysis was done using Kruskal-Wallis test with Dunn’s comparisons. (B) The 4-hour time point from the experiment depicted in panel A. Polyclonal CFP+ CD8 T cells (blue) and GFP+ OT-I cells (red) were followed in the BM after administration of OVA–Alexa 594 (yellow). Cell tracks (white) indicate continued motility of polyclonal cells (left), while OT-I cells arrest (right). Bars represent 50 µm. See also supplemental Video 4. (C) Three-dimensional paths of individual polyclonal CD8+ T and OT-I cells 4 hours after injection of OVA showing shorter paths for OT-I cells. (D) CFP+ polyclonal CD8+ T cells (blue) and CMTMR-labeled OT-I T cells (yellow) were transferred into a mouse harboring Cx3cr1-GFP+ cells (green) that had been injected with 0.5 mg OVA 18 hours before. T cells were analyzed 4 hours after transfer. The BM microvasculature was visualized by Quantum dots (red). Data are from 1 representative experiment of 3. Unlike polyclonal T cells, OT-I cells cluster in the vicinity of GFP+ cells. Bar represents 50 µm. See also supplemental Video 5. (E) T-cell activation in various lymphoid organs following challenge with blood-borne antigen. Wild-type C57BL/6 CD45.2 mice were transferred with OT-I CD45.1 naïve T cells and challenged intravenously with 0.5 mg OVA protein or served as controls. The bars represent the percentage of CD69+ T cells (gated as in supplemental Figure 2) in the BM, spleen, and LNs 2 to 3 hours after OVA injection, as determined by flow cytometry. Repeated-measure ANOVA (n = 7) indicated a significant effect of OVA treatment (P < .0001) and of the organ of origin (P < .0001) and a significant interaction (P < .0001). Scheffé’s post hoc comparisons indicated that OVA challenge increased T-cell activation in all organs (P < .0001 for all) and that T-cell activation was greater in the BM than in the spleen and LNs, both at the steady state and after OVA challenge (P < .001 for all). T-cell activation was greater in the spleen than in LN after OVA challenge (P < .001) but not at steady state (P = .65). Data are from 2 independent experiments. Asterisk denotes significant differences; error bars represent SEM. (F) Proliferation of OT-I T cells in the BM following OVA challenge. Wild-type mice were transferred with OT-I cells injected with 0.1 mg OVA 18 hours later and analyzed 48 hours later. Representative FACS histograms show CFSE dilution in CD45.1 OT-I cells; data are from 1 representative experiment out of 5. (G) Levels of Ki-67 (an early indicator of proliferation) in OT-I T cells retrieved from the spleen, BM, and blood of mice at various times after challenge with 0.1 mg OVA. The lines follow the percentage of Ki-67+ cells (non-G0 cells) among OT-I cells. Ki-67 levels in the BM and spleen showed a similar pattern, rising by 24 hours and plateauing at 36 hours, while blood levels lagged by a day. Error bars represent SEM. (H) The frequency of CD69− and CD69+ OT-I cells in the blood, spleen, and BM of the host mice analyzed in panel G. Curves show the percentage of CD69+ OT-I cells out of CD8+ cells stacked on top of the percentage of CD69− OT-I cells. Blood levels of CD69+ OT-I cells remained low. While CD69+ cells accumulated gradually in the spleen and BM, these cells downregulated CD69 only 48 hours after antigen challenge. (I) Snapshots from 2pm movies (supplemental Videos 6 and 7) show transferred GFP+ OT-I cells (green) in mice injected (right) or not (left) with 0.1 mg OVA 48 hours before. Blood sinusoids appear red. Cell volume increased and, as shown in the insets, cells divided locally in the BM in response to blood-borne antigen. Data are from 1 representative experiment of 2. Bar represents 50 µm. SP, spleen.
Naïve CD8+ T cells are efficiently activated in the BM upon antigenic challenge. (A) Average velocities of transferred polyclonal CFP+ CD8+ T cells and GFP+ OT-I cells, collected from the spleen and LNs, in the presence of antigenic protein. During BM imaging, the mice were challenged with 0.1 mg OVA–Alexa 594 and were continuously imaged for 5 hours. Cell speed gradually decreased in an antigen-specific manner. Data points represent individual cells taken from 1 representative experiment out of 3. *P < .05; **P < .001 (based on a comparison between polyclonal and OT-I T cells within the same time point). Multiple comparisons analysis was done using Kruskal-Wallis test with Dunn’s comparisons. (B) The 4-hour time point from the experiment depicted in panel A. Polyclonal CFP+ CD8 T cells (blue) and GFP+ OT-I cells (red) were followed in the BM after administration of OVA–Alexa 594 (yellow). Cell tracks (white) indicate continued motility of polyclonal cells (left), while OT-I cells arrest (right). Bars represent 50 µm. See also supplemental Video 4. (C) Three-dimensional paths of individual polyclonal CD8+ T and OT-I cells 4 hours after injection of OVA showing shorter paths for OT-I cells. (D) CFP+ polyclonal CD8+ T cells (blue) and CMTMR-labeled OT-I T cells (yellow) were transferred into a mouse harboring Cx3cr1-GFP+ cells (green) that had been injected with 0.5 mg OVA 18 hours before. T cells were analyzed 4 hours after transfer. The BM microvasculature was visualized by Quantum dots (red). Data are from 1 representative experiment of 3. Unlike polyclonal T cells, OT-I cells cluster in the vicinity of GFP+ cells. Bar represents 50 µm. See also supplemental Video 5. (E) T-cell activation in various lymphoid organs following challenge with blood-borne antigen. Wild-type C57BL/6 CD45.2 mice were transferred with OT-I CD45.1 naïve T cells and challenged intravenously with 0.5 mg OVA protein or served as controls. The bars represent the percentage of CD69+ T cells (gated as in supplemental Figure 2) in the BM, spleen, and LNs 2 to 3 hours after OVA injection, as determined by flow cytometry. Repeated-measure ANOVA (n = 7) indicated a significant effect of OVA treatment (P < .0001) and of the organ of origin (P < .0001) and a significant interaction (P < .0001). Scheffé’s post hoc comparisons indicated that OVA challenge increased T-cell activation in all organs (P < .0001 for all) and that T-cell activation was greater in the BM than in the spleen and LNs, both at the steady state and after OVA challenge (P < .001 for all). T-cell activation was greater in the spleen than in LN after OVA challenge (P < .001) but not at steady state (P = .65). Data are from 2 independent experiments. Asterisk denotes significant differences; error bars represent SEM. (F) Proliferation of OT-I T cells in the BM following OVA challenge. Wild-type mice were transferred with OT-I cells injected with 0.1 mg OVA 18 hours later and analyzed 48 hours later. Representative FACS histograms show CFSE dilution in CD45.1 OT-I cells; data are from 1 representative experiment out of 5. (G) Levels of Ki-67 (an early indicator of proliferation) in OT-I T cells retrieved from the spleen, BM, and blood of mice at various times after challenge with 0.1 mg OVA. The lines follow the percentage of Ki-67+ cells (non-G0 cells) among OT-I cells. Ki-67 levels in the BM and spleen showed a similar pattern, rising by 24 hours and plateauing at 36 hours, while blood levels lagged by a day. Error bars represent SEM. (H) The frequency of CD69− and CD69+ OT-I cells in the blood, spleen, and BM of the host mice analyzed in panel G. Curves show the percentage of CD69+ OT-I cells out of CD8+ cells stacked on top of the percentage of CD69− OT-I cells. Blood levels of CD69+ OT-I cells remained low. While CD69+ cells accumulated gradually in the spleen and BM, these cells downregulated CD69 only 48 hours after antigen challenge. (I) Snapshots from 2pm movies (supplemental Videos 6 and 7) show transferred GFP+ OT-I cells (green) in mice injected (right) or not (left) with 0.1 mg OVA 48 hours before. Blood sinusoids appear red. Cell volume increased and, as shown in the insets, cells divided locally in the BM in response to blood-borne antigen. Data are from 1 representative experiment of 2. Bar represents 50 µm. SP, spleen.
When the order of T-cell and antigen transfer was reversed and mice received 0.5 mg OVA protein 18 hours before T-cell transfer, OT-I cells visibly clustered in the vicinity of CX3CR1-GFP+ cells, whereas polyclonal T cells remained scattered (Figure 3D; supplemental Video 5). We suspect that T cells cluster as they exit the blood sinusoids at restricted areas and stop as soon as they encounter a cell presenting the antigen.
Next, we investigated whether, as T cells decelerated, they also acquired the early activation marker CD69. We transferred OT-I T cells (CD45.1) into wild-type recipients (CD45.2) and 18 hours later injected the mice intravenously with 0.5 mg OVA protein. Two hours later, before activated T cells could have migrated between organs, the activation status of OT-I cells was assessed in the spleen, LNs, and BM. Naïve OT-I cells in the BM expressed higher basal levels of CD69 than in the spleen or LNs (Figure 3E; supplemental Figure 2). After antigen challenge, the proportion of OT-I cells expressing CD69 increased significantly in all lymphoid organs, with the BM showing the highest expression levels, followed by the spleen and then the LNs, which have limited access to blood-borne antigens. These results suggest that BM-resident T cells are semiactivated and that CD69 may promote T-cell retention in the BM early after activation.
To study subsequent stages of T-cell activation leading to protective immunity, we investigated T-cell proliferation. We adoptively transferred CD45.1 OT-I cells labeled with CFSE and challenged the recipient mice with 0.1 mg OVA. Flow cytometric analysis 48 hours later revealed that T cells in the BM had vigorously proliferated (Figure 3F).
To ensure that T cells have detected antigen and divided locally rather than migrated as activated cells from other organs, we studied the Ki-67 protein, an early indicator of cell proliferation. Ki-67 levels in BM OT-I T cells rose within 24 hours, with no delay compared with the spleen (Figure 3G). To further assess whether migration from the spleen was a likely source for proliferating BM T cells, we followed OT-I cell numbers and CD69 expression in the blood, spleen, and BM for 48 hours (Figure 3H). OT-I cells quickly disappeared from the blood and reappeared there only after 48 hours, when their CD69 levels had dropped. Numbers of CD69− cells in the spleen remained low up to 48 hours, long after Ki-67+ T cells had been detected in the BM. In keeping with the established role of CD69 in T-cell egress,31 these findings suggest that in the first 36 hours after exposure to the antigen, while T cells express high membranal CD69, there is no major T-cell migration from the spleen through the blood to the BM.
To directly visualize local T-cell proliferation in the BM, we used 2PM. By 48 hours, T cells had become blasts displaying increased volume (1400 vs 925 μm3, P = .002, n = 64, 51) and crawled faster (8.6 vs 5 μm/min, P < .0001, n = 127, 235) (Figure 3I; supplemental Video 6). In more than 10 instances, we could directly observe large immobile cells dividing in the BM cavities. The cells completed cytokinesis in ∼4 minutes (Figure 3I right panel; supplemental Video 7).
Overall, these results indicate that in the BM, several APC populations sample blood-borne antigens and that naïve CD8+ T cells respond to their presentation by arrest, activation, and in situ proliferation, exhibiting an equivalent response to the splenic one.
Activation of naïve CD8+ T cells in the BM does not depend exclusively on DCs
DCs are generally believed to be the main, if not only, cells that crosspresent antigens and prime naïve CD8+ T cells.13,32,33 We therefore focused first on DCs as potential crosspresenters in the BM. We have recently shown that DCs cluster in distinct perivascular niches in the BM.15 To further define this population in relation to DCs in other organs, we investigated its origin by transferring established DC precursors into DC-depleted mice. Using DTx, we depleted CD11c-DTRtg mice of CD11chi DCs. Fluorescence-activated cell sorting (FACS) analysis verified that, as previously reported,15 DCs were efficiently ablated from the BM (Figure 4A). Examining the morphology of residual GFP+ cells in the BM CD11c-DTRtg×Cx3cr1gfp/+–depleted mice revealed that round monocytes and fusiform perivascular macrophages were spared whereas branched DCs had disappeared (Figure 4B). Flow cytometric analysis of pre-DCs, the immediate precursors of classical DCs,34 revealed that these cells were mostly spared from ablation as they lacked expression of the DTR-GFP fusion protein (Figure 4A).
DCs develop in the BM from MDPs without monocytic intermediates and are not essential for activation of naïve CD8+ T cells. (A) Flow cytometry of BM cells from wild-type mice reconstituted with BM from CD11c-DTRtg mice. Chimeric mice were left untreated or received an intraperitoneal injection of DTx. Twenty-four hours after treatment, the percentage of CD11chi MHC-II+ DCs and Lin− CD11chi MHCII- Flt3+ SIRPα− pre-DCs was calculated. Numbers adjacent to outlined areas indicate DC or pre-DC percentage. Data are from 1 representative experiment out of 3. (B) Images of the cranial BM cavities of [CD11c-DTRtg×Cx3cr1gfp/+ into wild-type] chimeric mice treated (right) or not (left) with DTx. The treatment thoroughly depletes DCs but spares round monocytes (middle) and spindle-shaped macrophages (right). GFP+ cells appear green and blood vessels appear red. Data are from 1 representative experiment of 3. Bars represent 10, 10, and 25 µm. (C) BM images of DTx-treated CD11c-DTRtg into wild-type chimeric mice after transfer of monocytes or MDPs. Fourteen days after transfer, monocytes did not give rise to DCs (left) while MDPs efficiently generated DCs in the BM (right). Data are from 1 representative experiment of 2. Bars represent 40 µm. (D) Chimeric mice (CD11c-DTRtg×Cx3cr1gfp/+ into wild-type) were treated with DTx and injected with 0.5 mg OVA. Sixteen hours later, mice were transferred with CMTMR-labeled OT-I T cells (yellow) and CFP+ polyclonal CD8+ T cells (blue) to be imaged 4 hours later using 2pm. Despite DC depletion, OT-I cells formed antigen-dependent clusters (indicated). Green cells are monocytes spared in the BM after DC ablation. Data are from 1 representative experiment of 2. Bar represents 50 µm. See also supplemental Video 8. (E) Velocities of polyclonal and OT-I CD8+ T cells transferred into chimeric mice that were injected with 0.5 mg OVA and either treated or not with DTx as described in panel A. Data points represent individual cells. The mean velocity of polyclonal T cells and OT-I cells in non–DTx-treated mice was 8.7 vs 3.8 μm/min (n = 40 and 67, respectively); in DTx-treated mice, the mean velocity was 5.7 vs 2.47 μm/min (n = 45 and 37). Individual 2-tailed t tests compared OT-I and polyclonal T cells within each treatment. Data are pooled from 3 untreated mice and 2 DTx-treated mice. *P < .001. (F) Proliferation of OT-I T cells in DC-depleted or intact mice. Chimeric mice (CD11c-DTRtg into wild-type) were treated or not with DTx and transferred with CFSE-labeled CD45.1 OT-I cells. Sixteen hours later, the mice were injected or not with 0.1 mg OVA. Forty-eight hours later, T-cell division in the BM, spleen, and LNs was analyzed using CFSE dilution. Histograms represent transferred cells gated based on CD45.1 and CD8 expression. (G) Analysis of proliferative capacity. The bars represent DI calculated from pooled data from 2 independent experiments involving 5 mice in each immunization condition (for which representative plots are shown in panel F). DC depletion abrogated proliferation in the spleen (DI of 0.3) but not in the BM (DI of 1.6). *P < .05; **P < .01; ***P < .001. Two-way ANOVA was used to compare, within each organ, OVA-treated mice with either untreated or DTx and OVA-treated mice. Error bars represent SEM. SP, spleen.
DCs develop in the BM from MDPs without monocytic intermediates and are not essential for activation of naïve CD8+ T cells. (A) Flow cytometry of BM cells from wild-type mice reconstituted with BM from CD11c-DTRtg mice. Chimeric mice were left untreated or received an intraperitoneal injection of DTx. Twenty-four hours after treatment, the percentage of CD11chi MHC-II+ DCs and Lin− CD11chi MHCII- Flt3+ SIRPα− pre-DCs was calculated. Numbers adjacent to outlined areas indicate DC or pre-DC percentage. Data are from 1 representative experiment out of 3. (B) Images of the cranial BM cavities of [CD11c-DTRtg×Cx3cr1gfp/+ into wild-type] chimeric mice treated (right) or not (left) with DTx. The treatment thoroughly depletes DCs but spares round monocytes (middle) and spindle-shaped macrophages (right). GFP+ cells appear green and blood vessels appear red. Data are from 1 representative experiment of 3. Bars represent 10, 10, and 25 µm. (C) BM images of DTx-treated CD11c-DTRtg into wild-type chimeric mice after transfer of monocytes or MDPs. Fourteen days after transfer, monocytes did not give rise to DCs (left) while MDPs efficiently generated DCs in the BM (right). Data are from 1 representative experiment of 2. Bars represent 40 µm. (D) Chimeric mice (CD11c-DTRtg×Cx3cr1gfp/+ into wild-type) were treated with DTx and injected with 0.5 mg OVA. Sixteen hours later, mice were transferred with CMTMR-labeled OT-I T cells (yellow) and CFP+ polyclonal CD8+ T cells (blue) to be imaged 4 hours later using 2pm. Despite DC depletion, OT-I cells formed antigen-dependent clusters (indicated). Green cells are monocytes spared in the BM after DC ablation. Data are from 1 representative experiment of 2. Bar represents 50 µm. See also supplemental Video 8. (E) Velocities of polyclonal and OT-I CD8+ T cells transferred into chimeric mice that were injected with 0.5 mg OVA and either treated or not with DTx as described in panel A. Data points represent individual cells. The mean velocity of polyclonal T cells and OT-I cells in non–DTx-treated mice was 8.7 vs 3.8 μm/min (n = 40 and 67, respectively); in DTx-treated mice, the mean velocity was 5.7 vs 2.47 μm/min (n = 45 and 37). Individual 2-tailed t tests compared OT-I and polyclonal T cells within each treatment. Data are pooled from 3 untreated mice and 2 DTx-treated mice. *P < .001. (F) Proliferation of OT-I T cells in DC-depleted or intact mice. Chimeric mice (CD11c-DTRtg into wild-type) were treated or not with DTx and transferred with CFSE-labeled CD45.1 OT-I cells. Sixteen hours later, the mice were injected or not with 0.1 mg OVA. Forty-eight hours later, T-cell division in the BM, spleen, and LNs was analyzed using CFSE dilution. Histograms represent transferred cells gated based on CD45.1 and CD8 expression. (G) Analysis of proliferative capacity. The bars represent DI calculated from pooled data from 2 independent experiments involving 5 mice in each immunization condition (for which representative plots are shown in panel F). DC depletion abrogated proliferation in the spleen (DI of 0.3) but not in the BM (DI of 1.6). *P < .05; **P < .01; ***P < .001. Two-way ANOVA was used to compare, within each organ, OVA-treated mice with either untreated or DTx and OVA-treated mice. Error bars represent SEM. SP, spleen.
To study the origin of BM-resident DCs, we transferred either monocytes or macrophage/DC precursors (MDPs) into the DTx-treated mice. While monocytes did not give rise to DCs in the BM, MDPs efficiently reconstituted the DCs (Figure 4C). In this respect, the DC population in the BM resembles classical DCs in the LNs and spleen34 rather than DCs in the periphery.
To determine whether DCs in the BM, like their splenic counterparts,13 are the only cells in the organ capable of activating naïve CD8+ T cells, we tested T-cell responses to antigen challenge in CD11c-DTRtg into wild-type chimeric mice treated with DTx. Single systemic DTx injection resulted in persistent DC ablation in the spleen and in the BM (supplemental Figure 3). Surprisingly, in the presence of antigen, OT-I cells still aggregated (Figure 4D; supplemental Video 8) and decelerated compared with polyclonal T cells (2.47 vs 5.7 μm/min, P < .001) (Figure 4E).
In view of these observations, we investigated whether DCs are needed to trigger CD8+ T cell proliferation. We used the proliferation assay described before in mice treated or not with DTx (injected twice to ensure persistent depletion). Transferred T cells readily proliferated in the spleen and BM of DC-proficient mice, yielding a high division index (DI) of 2.2 and 2, respectively (Figure 4F-G). As reported previously,13 DC depletion abrogated proliferation in the spleen (DI of 0.3) but, in accordance with our imaging data, not in the BM (DI of 1.6). It is possible that residual OT-I memory cells were the ones that proliferated as well as that transferred CD8+ DCs could present the antigen in DTx-treated mice. To rule out these options, we repeated the previous experiment using highly enriched naïve CD8+ T cells, which were magnetically selected for CD8α expression and depleted of CD11c+ and CD44+ cells using FACS. The response of the highly enriched T cells (supplemental Figure 4) resembled the one observed following regular enrichment (Figure 4F-G), indicating that in both cases endogenous APCs activated naïve T cells.
Residual DCs that might have survived after incomplete depletion could have driven T-cell proliferation. To exclude this possibility, we tested the T-cell response to an antigen targeted specifically to DCs. DC inhibitory receptor-2, which is recognized by the 33D1 monoclonal antibody,35 is expressed on most DCs in the BM (supplemental Figure 5A). Accordingly, treatment of mice with OVA conjugated to anti-33D1 (12 µg) resulted in a robust T-cell response. Depleting CD11c-DTRtg into wild-type chimeric mice of DCs abolished the T-cell response to 33D1-OVA but not to free OVA, confirming that DC ablation was complete (supplemental Figure 5B). Thus, apart from BM-resident DCs, other APCs likely crosspresented and activated naïve CD8+ T cells in the BM, driving the first steps of a T-cell response.
Taken together, our data suggest that, on top of DCs, the BM contains other APCs that can stimulate naïve CD8+ T cells to arrest, cluster, and proliferate in situ.
Hematopoietic rather than stromal cells crosspresent blood-borne antigens to CD8 T cells in the BM
A handful of studies have demonstrated that nonhematopoietic cells, such as mesenchymal stromal cells36 and scavenger liver sinusoidal endothelial cells,37 can crosspresent exogenous antigen on MHC-I and stimulate naïve CD8+ T cells. Moreover, a recent report showed that CD8+ T cells in the lung could be primed by influenza-infected epithelial cells to become cytotoxic.38 Given that the BM is populated by a variety of stromal cells and that fluorescence from OVA–Alexa 594 eventually accumulated in endothelial-like cells (Figure 2A), we tested whether nonhematopoietic cells could account for the CD8 T-cell activation. To this end, we reconstituted lethally irradiated H-2Kb–deficient mice19 with BM from CD11c-DTRtg mice. The resulting chimeric mice contained hematopoietic cells that can present the SIINFEKL peptide on MHC-I along with radiation-resistant stromal cells, which cannot. In addition, DCs of these mice could be selectively depleted to study the role of other hematopoietic cells. Reciprocal (H-2Kb−/− into IL-15−/−) chimeric mice were generated to study whether stromal cells can crosspresent in the absence of functional hematopoietic cells. We used irradiated IL-15–deficient recipients20 because they lack natural killer cells that would otherwise reject the MHC-I–deficient graft. In H-2Kb−/− into IL-15−/− chimeric mice, in which only the stromal cells can present OVA peptide, grafted OT-I cells proliferated neither in the BM nor in the spleen (Figure 5A). In contrast, in CD11c-DTRtg into H-2Kb−/− mice, in which only hematopoietic cells could present, the T cells proliferated normally (Figure 5A; supplemental Figure 6). In agreement with our earlier findings, depleting these chimeric mice of DCs, allowing only other hematopoietic cells to present, blocked T-cell proliferation in the spleen but not in the BM (Figure 5A).
Activation of naïve CD8+ T cells in the BM does not depend on crosspresentation by stromal cells or B cells but is abrogated by ablation of monocytes, macrophages, and DCs using DTx and CLL. (A) Proliferation of OT-I T cells when either stromal or hematopoietic cells can crosspresent antigen. The indicated chimeric mice were transferred with CFSE-labeled CD45.1 OT-I cells, either treated or not with DTx, injected with 0.1 mg OVA, and analyzed by flow cytometry 48 hours later. Histograms represent CFSE profiles of cells gated according to CD45.1 and CD8 expression. In the BM, presentation was carried out by hematopoietic cells but not only DCs. Data are from 1 representative experiment of 3. (B) Proliferation of OT-I T cells in the absence of B cells. Flow cytometry of transferred OT-I cells into CD11c-DTRtg and CD11c-DTRtg×JH−/− mice treated as in (A). T cell proliferation in the BM persisted in the combined absence of B cells and DCs. Data are from 1 representative experiment of 2. (C) Depletion of mononuclear phagocytes using CLL. Flow cytometric analysis of monocytes (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II−), macrophages (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II+), DCs (CD11chi MHC-IIhi), pDCs (CD11cint mPDCA-1hi), and pre-DCs (Lin− CD11chi MHCII- Flt3+ SIRPα−) 1 day after intravenous injection of CLL. Bars represent total numbers of cells isolated from 2 tibiae and 2 femurs. CLL treatment efficiently depleted almost all BM monocytes and macrophages alongside with half of the DCs. Individual 2-tailed t tests were conducted for each cell type. *P < .05; **P < .0001, Error bars represent SEM. Data are from 1 representative experiment of 2 (n = 3 in each group). (D) Proliferation of OT-I T cells in the absence of mononuclear phagocytes. Chimeric mice [CD11c-DTRtg into wild-type] were transferred with CFSE-labeled CD45.1 OT-I cells; either treated or not with DTx, CLL, or both; injected with 0.1 mg OVA; and analyzed by flow cytometry 48 hours later. Histograms represent CFSE profiles of cells gated according to CD45.1 and CD8 expression. Data are from 1 representative experiment of 2. (E) Analysis of proliferative capacity. The bars represent DI calculated from pooled data from 2 independent experiments involving 2 to 6 mice in each condition (for which representative plots are shown in panel D). T-cell proliferation in the BM was abrogated when all mononuclear phagocytes were depleted. *P < .001. Two-way ANOVA was used to compare, within each organ, OVA-treated mice with each of the other treatment groups. Error bars represent SEM. (F) Images of GFP+ OT-I cells (green) transferred into OVA-injected CD11c-DTRtg into wild-type chimeric mice that had been left intact (left) or treated with DTx and CLL (right). T cells were analyzed by 2pm 48 hours after OVA challenge. The BM microvasculature was visualized by Quantum dots (red). T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were sparser and failed to divide. Data are from 1 representative experiment of 2. Bar represents 50 µm. See also supplemental Video 9. cDCs, conventional DCs.
Activation of naïve CD8+ T cells in the BM does not depend on crosspresentation by stromal cells or B cells but is abrogated by ablation of monocytes, macrophages, and DCs using DTx and CLL. (A) Proliferation of OT-I T cells when either stromal or hematopoietic cells can crosspresent antigen. The indicated chimeric mice were transferred with CFSE-labeled CD45.1 OT-I cells, either treated or not with DTx, injected with 0.1 mg OVA, and analyzed by flow cytometry 48 hours later. Histograms represent CFSE profiles of cells gated according to CD45.1 and CD8 expression. In the BM, presentation was carried out by hematopoietic cells but not only DCs. Data are from 1 representative experiment of 3. (B) Proliferation of OT-I T cells in the absence of B cells. Flow cytometry of transferred OT-I cells into CD11c-DTRtg and CD11c-DTRtg×JH−/− mice treated as in (A). T cell proliferation in the BM persisted in the combined absence of B cells and DCs. Data are from 1 representative experiment of 2. (C) Depletion of mononuclear phagocytes using CLL. Flow cytometric analysis of monocytes (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II−), macrophages (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II+), DCs (CD11chi MHC-IIhi), pDCs (CD11cint mPDCA-1hi), and pre-DCs (Lin− CD11chi MHCII- Flt3+ SIRPα−) 1 day after intravenous injection of CLL. Bars represent total numbers of cells isolated from 2 tibiae and 2 femurs. CLL treatment efficiently depleted almost all BM monocytes and macrophages alongside with half of the DCs. Individual 2-tailed t tests were conducted for each cell type. *P < .05; **P < .0001, Error bars represent SEM. Data are from 1 representative experiment of 2 (n = 3 in each group). (D) Proliferation of OT-I T cells in the absence of mononuclear phagocytes. Chimeric mice [CD11c-DTRtg into wild-type] were transferred with CFSE-labeled CD45.1 OT-I cells; either treated or not with DTx, CLL, or both; injected with 0.1 mg OVA; and analyzed by flow cytometry 48 hours later. Histograms represent CFSE profiles of cells gated according to CD45.1 and CD8 expression. Data are from 1 representative experiment of 2. (E) Analysis of proliferative capacity. The bars represent DI calculated from pooled data from 2 independent experiments involving 2 to 6 mice in each condition (for which representative plots are shown in panel D). T-cell proliferation in the BM was abrogated when all mononuclear phagocytes were depleted. *P < .001. Two-way ANOVA was used to compare, within each organ, OVA-treated mice with each of the other treatment groups. Error bars represent SEM. (F) Images of GFP+ OT-I cells (green) transferred into OVA-injected CD11c-DTRtg into wild-type chimeric mice that had been left intact (left) or treated with DTx and CLL (right). T cells were analyzed by 2pm 48 hours after OVA challenge. The BM microvasculature was visualized by Quantum dots (red). T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were sparser and failed to divide. Data are from 1 representative experiment of 2. Bar represents 50 µm. See also supplemental Video 9. cDCs, conventional DCs.
Because B cells could take up blood-borne OVA (Figure 2B), we next investigated their capacity to crosspresent it to T cells. To this end, we tested CD11c-DTRtg×JH−/− mice for their ability to mount CD8+ T-cell responses (Figure 5B). Neither the B-cell deficiency alone nor its combination with DC ablation interfered with T-cell proliferation in the BM in our system, excluding B cells as potential presenters.
Apart from DCs, key APCs that could activate naïve CD8+ T cells in the BM are the other mononuclear phagocytes: monocytes and macrophages. Indirect evidence suggests that both cell types can take part in such a process.39,40 To test the ability of BM-resident monocytes and macrophages to present antigens and activate T cells in situ, we used CLL, which efficiently depletes phagocytic cells.23 Systemic injection of CLL efficiently depleted almost all monocytes and macrophages alongside with approximately half of the DCs in the BM; pDCs and pre-DCs, in contrast, were mildly affected (Figure 5C). We tested OT-I proliferation in OVA-injected CD11c-DTRtg mice that had been treated with DTx and CLL, alone or in combination. As expected, depletion of DCs sufficed to abolish T-cell proliferation in the spleen. However, preventing proliferation in the BM required depletion of all phagocytic cells (Figure 5D-E). Indeed, by 2PM, T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes (Figure 5F right panel) were sparser than in intact mice (Figure 5F left panel) (439 ± 112, 1072 ± 170 cells/mm2, P < .001) and failed to divide (supplemental Video 9). We validated that these results were not due to direct toxicity of the combined treatment toward transferred T cells in the BM (supplemental Figure 7A); although T-cell numbers in the spleen were reduced, no direct effects were observed in the BM.
T cells can be primed following immunization with blood-borne antigen and adjuvant even in the absence of DCs
So far, we investigated early events in T-cell activation, which can result in either priming or tolerance, depending on the inflammatory context. Indeed, systemic administration of antigen alone is usually tolerogenic.41 To test whether these early events in the BM can initiate a productive immune response, we employed the TLR9 ligand CpG as an adjuvant.42
To validate that our findings regarding early activation events in the BM hold true also in this inflammatory context, we made the following observations. Imaging of the BM following intravenous coinjection of labeled OVA with CpG (Figure 6A) showed a pattern of uptake indiscernible from OVA alone (Figure 2A): antigen could be seen in CX3CR1-GFP+ cells at 1 hour and remained only in endothelial or perivascular cells the following day. FACS analysis indicated that the same populations of cells took up the antigen but the phagocytic capacity was slightly increased, especially in B cells (Figure 6B). Regardless of DC depletion, T cells clustered (Figure 6C; supplemental Video 10) and decelerated in an antigen-specific manner (Figure 6D). T-cell proliferation (Figure 6E) closely resembled the proliferation obtained with OVA alone and responded similarly to depletion of DCs (Figure 4G). The diminished T-cell response after combined depletion led to visibly sparser OT-I T cells in the BM (Figure 6F-G), which were not observed dividing (Figure 6F).
T-cell priming following immunization with blood-borne antigen and adjuvant. (A) Images of in vivo antigen uptake by BM cells of a Cx3cr1gfp/+ mouse at different time points after injection of 0.5 mg fluorescently tagged OVA (yellow) and 10 nmol CpG. An hour after injection, the antigen had been taken up by many cell types, including green CX3CR1-GFP+ cells (arrows, insets, middle). After 24 hours, the fluorescent signal (arrows, right) persisted only in flat cells that line blood vessels (red), consistent with endothelial or perivascular cells (insets, right). Data are from 1 representative experiment of 2. Bar represents 50 µm. (B) Flow cytometric analysis of untreated BM or BM harvested 2 hours after intravenous injection of 0.5 mg fluorescently tagged OVA–Texas red with or without CpG. Histograms depict OVA acquisition, as indicated by the Texas red signal in pDCs (CD11cint mPDCA-1hi), T cells (CD3hi), B cells (CD19hi), conventional DCs (cDCs) (CD11chi MHC-II+), macrophages (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II+), Ly6Clo monocytes (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II−), and Ly6Chi monocytes (CX3CR1-GFPhi, CD11c−). Phagocytic capacity was slightly increased in CpG-treated mice, especially in the case of B cells. (C) Chimeric mice (CD11c-DTRtg×Cx3cr1gfp/+ into wild-type) were pretreated with DTx (right) or not (left) and then immunized with 0.5 mg OVA and 10 nmol CpG. Sixteen hours later, mice were transferred with GFP OT-I T cells (green) and CFP+ polyclonal CD8+ T cells (blue) to be imaged 4 hours later using 2PM. Regardless of DC depletion, OT-I cells formed antigen-dependent clusters. See also supplemental Video 10. Bar represents 50 µm. (D) Velocities of polyclonal and OT-I CD8+ T cells transferred into chimeric mice that had been injected with OVA + CpG and either treated or not with DTx as described in panel C. Data points represent individual cells. The mean velocity of polyclonal T cells and OT-I cells in untreated mice was 5.9 vs 2.8 μm/min (n = 51 and 54, respectively); in DTx-treated mice, the mean velocity was 6.4 vs 3.4 μm/min, (n = 63 and 74, respectively). Regardless of DC depletion, OT-I cells decelerated. Individual 2-tailed t tests compared OT-I and polyclonal T cells within each experimental condition. *P < .001. (E) Analysis of proliferative capacity following immunization with 0.5 mg OVA + CpG. The bars represent DI calculated from pooled data from 2 independent experiments involving 5 mice in each immunization condition. DC depletion significantly reduced proliferation in the spleen (DI of 0.6) but not in the BM (DI of 1.6). *P < .001. Two-way ANOVA was used to compare, within each organ, OVA + CpG–treated mice with the 2 other groups. Error bars represent SEM. (F) Images of GFP+ OT-I cells (green) transferred into OVA + CpG–injected [CD11c-DTRtg into wild-type] chimeric mice that had been left intact (left) or treated with DTx and CLL (right). T cells were analyzed by 2PM 48 hours after OVA challenge. T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were sparser and failed to divide. Insets depict a dividing cell. See also supplemental Video 11. Blood vessels were counterstained in red. Bar represents 50 µm. (G). Densities of OT-I cells in the BM, as calculated from several fields such as the ones depicted in panel F. T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were significantly sparser. *P < .01. Error bars represent SEM. (H) In vivo killing ability of OT-I CTLs primed in the presence or absence of mononuclear phagocytes. 5000 Naïve OT-I -CFP T cells were transferred into CD11c-DTRtg into wild-type chimeric mice prior to treatment with DTx either with or without CLL. Mice were then immunized with 0.5 mg OVA and 10 nmol CpG. Eight days later, recipient mice were injected with equal numbers of CD45.1 CFSEhi splenocytes pulsed with OVA peptide and CFSElo unpulsed cells. Antigen-specific killing was tested in the BM and spleens of recipient mice 1 day later. Combined depletion, but not DC depletion alone, annihilated target cell killing in both the spleen and BM. Bars show normalized percentage specific killing. *P < .05; **P < .01; ***P < .001. A 2-way ANOVA was used to compare, within each organ, OVA + CpG–treated mice with the 3 other groups. Error bars represent SEM. cDCs, conventional DCs; SP, spleen.
T-cell priming following immunization with blood-borne antigen and adjuvant. (A) Images of in vivo antigen uptake by BM cells of a Cx3cr1gfp/+ mouse at different time points after injection of 0.5 mg fluorescently tagged OVA (yellow) and 10 nmol CpG. An hour after injection, the antigen had been taken up by many cell types, including green CX3CR1-GFP+ cells (arrows, insets, middle). After 24 hours, the fluorescent signal (arrows, right) persisted only in flat cells that line blood vessels (red), consistent with endothelial or perivascular cells (insets, right). Data are from 1 representative experiment of 2. Bar represents 50 µm. (B) Flow cytometric analysis of untreated BM or BM harvested 2 hours after intravenous injection of 0.5 mg fluorescently tagged OVA–Texas red with or without CpG. Histograms depict OVA acquisition, as indicated by the Texas red signal in pDCs (CD11cint mPDCA-1hi), T cells (CD3hi), B cells (CD19hi), conventional DCs (cDCs) (CD11chi MHC-II+), macrophages (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II+), Ly6Clo monocytes (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II−), and Ly6Chi monocytes (CX3CR1-GFPhi, CD11c−). Phagocytic capacity was slightly increased in CpG-treated mice, especially in the case of B cells. (C) Chimeric mice (CD11c-DTRtg×Cx3cr1gfp/+ into wild-type) were pretreated with DTx (right) or not (left) and then immunized with 0.5 mg OVA and 10 nmol CpG. Sixteen hours later, mice were transferred with GFP OT-I T cells (green) and CFP+ polyclonal CD8+ T cells (blue) to be imaged 4 hours later using 2PM. Regardless of DC depletion, OT-I cells formed antigen-dependent clusters. See also supplemental Video 10. Bar represents 50 µm. (D) Velocities of polyclonal and OT-I CD8+ T cells transferred into chimeric mice that had been injected with OVA + CpG and either treated or not with DTx as described in panel C. Data points represent individual cells. The mean velocity of polyclonal T cells and OT-I cells in untreated mice was 5.9 vs 2.8 μm/min (n = 51 and 54, respectively); in DTx-treated mice, the mean velocity was 6.4 vs 3.4 μm/min, (n = 63 and 74, respectively). Regardless of DC depletion, OT-I cells decelerated. Individual 2-tailed t tests compared OT-I and polyclonal T cells within each experimental condition. *P < .001. (E) Analysis of proliferative capacity following immunization with 0.5 mg OVA + CpG. The bars represent DI calculated from pooled data from 2 independent experiments involving 5 mice in each immunization condition. DC depletion significantly reduced proliferation in the spleen (DI of 0.6) but not in the BM (DI of 1.6). *P < .001. Two-way ANOVA was used to compare, within each organ, OVA + CpG–treated mice with the 2 other groups. Error bars represent SEM. (F) Images of GFP+ OT-I cells (green) transferred into OVA + CpG–injected [CD11c-DTRtg into wild-type] chimeric mice that had been left intact (left) or treated with DTx and CLL (right). T cells were analyzed by 2PM 48 hours after OVA challenge. T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were sparser and failed to divide. Insets depict a dividing cell. See also supplemental Video 11. Blood vessels were counterstained in red. Bar represents 50 µm. (G). Densities of OT-I cells in the BM, as calculated from several fields such as the ones depicted in panel F. T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were significantly sparser. *P < .01. Error bars represent SEM. (H) In vivo killing ability of OT-I CTLs primed in the presence or absence of mononuclear phagocytes. 5000 Naïve OT-I -CFP T cells were transferred into CD11c-DTRtg into wild-type chimeric mice prior to treatment with DTx either with or without CLL. Mice were then immunized with 0.5 mg OVA and 10 nmol CpG. Eight days later, recipient mice were injected with equal numbers of CD45.1 CFSEhi splenocytes pulsed with OVA peptide and CFSElo unpulsed cells. Antigen-specific killing was tested in the BM and spleens of recipient mice 1 day later. Combined depletion, but not DC depletion alone, annihilated target cell killing in both the spleen and BM. Bars show normalized percentage specific killing. *P < .05; **P < .01; ***P < .001. A 2-way ANOVA was used to compare, within each organ, OVA + CpG–treated mice with the 3 other groups. Error bars represent SEM. cDCs, conventional DCs; SP, spleen.
Overall, the presence of the TLR-ligand CpG does not alter indicators of early T-cell activation appreciably; we therefore proceeded to examine in vivo cytotoxic T lymphocyte (CTL)-mediated lysis.
In line with the results of the early activation, ablation of DCs did not abolish target cell clearance (Figure 6H; supplemental Figure 7B-C), especially when the number of transferred OT-I cells was high (supplemental Figure 7B). However, unlike with earlier indices of T-cell activation, the spleen was also effectively cleared of target cells. This spread of T-cell function may have resulted from BM-generated CTLs that had migrated to the spleen during the intervening days. Combined treatment with DTx and CLL prevented target cell killing altogether. In vivo cytotoxicity in the various mouse groups was mirrored closely by the eventual frequency of OT-I CTLs in the BM and spleen (supplemental Figure 7D).
Overall, our data suggest that, in the BM, mononuclear phagocytes other than DCs can efficiently crosspresent blood-borne antigen, prime naïve CD8+ T cells, and drive a CTL response, distinguishing this organ from the spleen.
Discussion
In this report, we took advantage of 2PM and flow cytometry to examine 3 main questions: (1) Do naïve CD8+ T cells in the BM respond to soluble blood-borne antigens? (2) What are the consequences of such activation? (3) Which cells crosspresent the antigen to the CD8+ T cells?
Our study examined naïve CD8+ T cells and focused on the ability of endogenous APCs to capture and present the antigen acquired from the blood. As a consequence, we could follow the dynamics of antigen capture in the BM and suggest APC populations capable of crosspresentation.
We found that the BM contains a substantial population of naïve CD8+ T cells. These cells crawled vigorously in the BM cavities, exhibiting faster motility than B cells, and were somewhat preactivated, as indicated by elevated CD69 levels. After systemic injection of cognate peptide antigen, T cells responded to TCR ligation by immediate arrest.
Imaging, for the first time, the capture of an antigenic protein diffusing from the blood into the BM, we showed that many cell types had access to it. Among them were endothelial or perivascular cells and hematopoietic cells. In contrast to their immediate reaction to antigenic peptide, it took T cells 3 hours to fully arrest following systemic administration of antigenic protein. This lag likely represents the time APCs required to process the intact antigen and load its peptides onto membranal MHC-I molecules. Following activation, CD8+ T cells formed clusters in the vicinity of DCs in the BM, identified as fluorescent cells in Cx3cr1gfp/+ mice. In parallel, T cells upregulated the early activation marker CD69. Within 48 hours, T cells blasted and started to proliferate. Three lines of evidence converge to suggest that most of these proliferating T cells had been exposed to the antigen locally rather than in secondary lymphoid organs such as the spleen: (1) T cells entered G1 in the BM as early as in the spleen and proliferated at least as vigorously; (2) T cells proliferated in the BM in the absence of DCs, while no proliferation occurred in the spleen; and (3) most directly, using intravital 2PM, we could for the first time observe blasting and division of CD8+ T cells in the BM. Correspondingly, it has been shown that in mice devoid of LNs and spleen, T cells can be activated in the BM.10 Still, we cannot exclude the option that some T cells had been activated in other organs before migrating to the BM and proliferating there.
Beyond initial activation, naïve T cells that have been primed in the BM under polarizing conditions effectively formed CTLs that spread through the body to clear target cells, not only in the BM but also in the spleen.
Using chimeric mice and specific strategies for cell depletion, we could show that among the cells that had ingested the antigen, only hematopoietic cells could crosspresent it to prime CD8+ T cells. In contrast to the spleen, where only DCs could crosspresent antigen and prime T cells, in the BM, 2 capable populations were identified. First and foremost were DCs; these cells originated without monocytic intermediates from MDPs and could capture antigen from the blood and present it. A second APC population consisted of monocytes/macrophages, which were shown here to capture the antigen. Combined depletion of both cell populations, but not of DCs alone, almost totally abolished CD8+ T-cell activation.
At steady state, monocytes and macrophages are not considered APCs capable of efficient crosspresentation. However, indirect evidence exists that, under certain conditions, both cell populations can participate in this process. Monocytes can take up antigens in the BM, retain them intracellularly, and migrate to the LNs, where they become nonclassical DCs and present them to CD4+ T cells.39 Moreover, spleen-derived activated monocytes can crosspresent antigens acquired by pinocytosis or by DEC205-mediated endocytosis as efficiently as DCs.43
Likewise, macrophages can crosspresent antigens in certain situations. Macrophages can collaborate with DCs in splenic crosspresentation.44 Recently, CD169+ macrophages were demonstrated to be essential for crosspresentation of antigens from dead tumor cells.40 Indeed, marginal zone macrophages, which are depleted in CD11c-DTRtg mice,45 could also play a role in crosspresentation in the spleen of intact mice in our study. This possibility is also in line with recent results46 showing that mice lacking zDC+ DCs can still resist Toxoplasma gondii and reject immunogenic tumors, whereas mice lacking CD11c+ cells (which include some macrophage populations) cannot. Most pertinently, it was recently shown that mononuclear phagocytes in the BM can crosspresent viral antigens delivered by neutrophils into the BM and activate naïve CD8 T cells there.47
A less likely cell type to crosspresent antigen in the BM is the pDC. Although these cells abound in the BM and are not depleted by in CD11c-DTRtg mice,48 they did not phagocytose OVA and were not depleted by CLL, which abolished BM crosspresentation.
It has long been noticed that posttraumatic removal of the spleen, in otherwise healthy adults, results in minimal increase in susceptibility to sepsis.49 Together with a handful of earlier reports, our study proposes the BM as a substitute site to the spleen where primary responses to blood-borne antigens can occur. In fact, although it harbors fewer naïve T cells than the spleen and does not exhibit the well-organized structure of classical secondary lymphoid organs, in some aspects the BM is superior in its response. It has been demonstrated that T cells in the BM show indices of increased activation9 and exhibit increased turnover50 compared with their splenic counterparts. In this report, we further show, based on CD69 expression, that naïve T cells in the BM are preactivated. We further demonstrate that the BM is also less restricted in the APC population capable of crosspresentation.
Overall, our results suggest that, although long overlooked, the BM supports crosspresentation of blood-borne antigens to naïve CD8+ T cells similar to the spleen and is unique in its incomplete reliance on DCs. Three nonexclusive options may account for this nonreliance. Possibly, T cells are more preactivated in the BM, and may require less costimulation for priming. Alternatively, the BM microenvironment, with its unique architecture and molecular composition, might promote cellular interactions, which are precluded in the strictly segregated spleen. Finally, the BM microenvironment may contain unique APCs, other than DCs, which are capable of efficient crossactivation of naïve cells. Although monocytes and macrophages seem promising candidates as such supplementary APCs, further analysis is needed to establish the exact identity of these cells and whether they are unique to the BM. Such APCs can potentially be targeted for vaccination purposes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Idit Shachar for discussions in the conception stage of the project, the staff of the Weizmann Animal Facility for dedicated animal care, and Eitan Ariel and Ayala Sharp for help with flow cytometry.
This work was supported by the Willner Family Leadership Institute, the Wolfson Family Charitable Trust, the Minerva Foundation, and the Israel Science Foundation (G.S.); by the Israel Science Foundation and the YeS/Yeda-Sela Center (S.J.); and by BayGene, the German Israel Foundation (GIF), the German Research Foundation (DU548/2-1), and ELAN (intramural funding Erlangen) (D.D.).
Authorship
Contribution: G.S. and S.J. conceived the project and designed experiments; I.M. and A.S. conducted experiments; V.K., O.T., and R.K. provided technical assistance; I.M. and A.S. analyzed the data and together with G.S. wrote the manuscript; N.v.R. developed and produced CLL; and D.D. produced anti–33D1-OVA and together with S.J. reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guy Shakhar, Department of Immunology, The Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: shakhar@weizmann.ac.il.
References
Author notes
I.M. and A.S. contributed equally to this study.


![Figure 4. DCs develop in the BM from MDPs without monocytic intermediates and are not essential for activation of naïve CD8+ T cells. (A) Flow cytometry of BM cells from wild-type mice reconstituted with BM from CD11c-DTRtg mice. Chimeric mice were left untreated or received an intraperitoneal injection of DTx. Twenty-four hours after treatment, the percentage of CD11chi MHC-II+ DCs and Lin− CD11chi MHCII- Flt3+ SIRPα− pre-DCs was calculated. Numbers adjacent to outlined areas indicate DC or pre-DC percentage. Data are from 1 representative experiment out of 3. (B) Images of the cranial BM cavities of [CD11c-DTRtg×Cx3cr1gfp/+ into wild-type] chimeric mice treated (right) or not (left) with DTx. The treatment thoroughly depletes DCs but spares round monocytes (middle) and spindle-shaped macrophages (right). GFP+ cells appear green and blood vessels appear red. Data are from 1 representative experiment of 3. Bars represent 10, 10, and 25 µm. (C) BM images of DTx-treated CD11c-DTRtg into wild-type chimeric mice after transfer of monocytes or MDPs. Fourteen days after transfer, monocytes did not give rise to DCs (left) while MDPs efficiently generated DCs in the BM (right). Data are from 1 representative experiment of 2. Bars represent 40 µm. (D) Chimeric mice (CD11c-DTRtg×Cx3cr1gfp/+ into wild-type) were treated with DTx and injected with 0.5 mg OVA. Sixteen hours later, mice were transferred with CMTMR-labeled OT-I T cells (yellow) and CFP+ polyclonal CD8+ T cells (blue) to be imaged 4 hours later using 2pm. Despite DC depletion, OT-I cells formed antigen-dependent clusters (indicated). Green cells are monocytes spared in the BM after DC ablation. Data are from 1 representative experiment of 2. Bar represents 50 µm. See also supplemental Video 8. (E) Velocities of polyclonal and OT-I CD8+ T cells transferred into chimeric mice that were injected with 0.5 mg OVA and either treated or not with DTx as described in panel A. Data points represent individual cells. The mean velocity of polyclonal T cells and OT-I cells in non–DTx-treated mice was 8.7 vs 3.8 μm/min (n = 40 and 67, respectively); in DTx-treated mice, the mean velocity was 5.7 vs 2.47 μm/min (n = 45 and 37). Individual 2-tailed t tests compared OT-I and polyclonal T cells within each treatment. Data are pooled from 3 untreated mice and 2 DTx-treated mice. *P < .001. (F) Proliferation of OT-I T cells in DC-depleted or intact mice. Chimeric mice (CD11c-DTRtg into wild-type) were treated or not with DTx and transferred with CFSE-labeled CD45.1 OT-I cells. Sixteen hours later, the mice were injected or not with 0.1 mg OVA. Forty-eight hours later, T-cell division in the BM, spleen, and LNs was analyzed using CFSE dilution. Histograms represent transferred cells gated based on CD45.1 and CD8 expression. (G) Analysis of proliferative capacity. The bars represent DI calculated from pooled data from 2 independent experiments involving 5 mice in each immunization condition (for which representative plots are shown in panel F). DC depletion abrogated proliferation in the spleen (DI of 0.3) but not in the BM (DI of 1.6). *P < .05; **P < .01; ***P < .001. Two-way ANOVA was used to compare, within each organ, OVA-treated mice with either untreated or DTx and OVA-treated mice. Error bars represent SEM. SP, spleen.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/2/10.1182_blood-2012-01-401265/4/m_193f4.jpeg?Expires=1769317944&Signature=DgRhxvw~w1rHFwsJC3ArWcS5WyWlTUzxV1SN563Hd~uhhiiFhjkksdVTu0TNcBdnS5Z12JxggtMnboRJA~ekklX5X9RXPelEFPGI8D74fdDtn91gxGHJza0OPeDr5fllEvgGFww825l6t~3W1IJ~Zm-jXUrJvsWp2e57MywbVXzUCD1ePjsU0qlCdv15GTl8aXACAwdtnBBo~O3aTetplnll5Vm3fcu33TPw5bbZuMD~g642QVsPNAsW~O6SFlJ9vqReuNgiRoLaXPAFilRERhN0jpqE5kZFpmDFE0PRAuSp1IySQLTf71UoFncXqcLL3izHWvkjbLA8TSzk80PO6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Activation of naïve CD8+ T cells in the BM does not depend on crosspresentation by stromal cells or B cells but is abrogated by ablation of monocytes, macrophages, and DCs using DTx and CLL. (A) Proliferation of OT-I T cells when either stromal or hematopoietic cells can crosspresent antigen. The indicated chimeric mice were transferred with CFSE-labeled CD45.1 OT-I cells, either treated or not with DTx, injected with 0.1 mg OVA, and analyzed by flow cytometry 48 hours later. Histograms represent CFSE profiles of cells gated according to CD45.1 and CD8 expression. In the BM, presentation was carried out by hematopoietic cells but not only DCs. Data are from 1 representative experiment of 3. (B) Proliferation of OT-I T cells in the absence of B cells. Flow cytometry of transferred OT-I cells into CD11c-DTRtg and CD11c-DTRtg×JH−/− mice treated as in (A). T cell proliferation in the BM persisted in the combined absence of B cells and DCs. Data are from 1 representative experiment of 2. (C) Depletion of mononuclear phagocytes using CLL. Flow cytometric analysis of monocytes (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II−), macrophages (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II+), DCs (CD11chi MHC-IIhi), pDCs (CD11cint mPDCA-1hi), and pre-DCs (Lin− CD11chi MHCII- Flt3+ SIRPα−) 1 day after intravenous injection of CLL. Bars represent total numbers of cells isolated from 2 tibiae and 2 femurs. CLL treatment efficiently depleted almost all BM monocytes and macrophages alongside with half of the DCs. Individual 2-tailed t tests were conducted for each cell type. *P < .05; **P < .0001, Error bars represent SEM. Data are from 1 representative experiment of 2 (n = 3 in each group). (D) Proliferation of OT-I T cells in the absence of mononuclear phagocytes. Chimeric mice [CD11c-DTRtg into wild-type] were transferred with CFSE-labeled CD45.1 OT-I cells; either treated or not with DTx, CLL, or both; injected with 0.1 mg OVA; and analyzed by flow cytometry 48 hours later. Histograms represent CFSE profiles of cells gated according to CD45.1 and CD8 expression. Data are from 1 representative experiment of 2. (E) Analysis of proliferative capacity. The bars represent DI calculated from pooled data from 2 independent experiments involving 2 to 6 mice in each condition (for which representative plots are shown in panel D). T-cell proliferation in the BM was abrogated when all mononuclear phagocytes were depleted. *P < .001. Two-way ANOVA was used to compare, within each organ, OVA-treated mice with each of the other treatment groups. Error bars represent SEM. (F) Images of GFP+ OT-I cells (green) transferred into OVA-injected CD11c-DTRtg into wild-type chimeric mice that had been left intact (left) or treated with DTx and CLL (right). T cells were analyzed by 2pm 48 hours after OVA challenge. The BM microvasculature was visualized by Quantum dots (red). T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were sparser and failed to divide. Data are from 1 representative experiment of 2. Bar represents 50 µm. See also supplemental Video 9. cDCs, conventional DCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/2/10.1182_blood-2012-01-401265/4/m_193f5.jpeg?Expires=1769317944&Signature=jT-ZawYtq-Bh7N1YB~TsEe2nDcAd9gcUfQpzHmA74zlxonC8VPEVKATuRbmi22hQSlc0H~zy~lqhtFPKS9qL7wWhoUix3nFVC3nh5yjyypKeOaglgUfUTJdVT-dovxjaBa3o4fKlDkdUxikCbB0RtGy~LzVImekfaN5ms0asj2bFQsgsNyMoiEqEKiN0Gon5f6c9P7x3hysXUnaZxILAhhlQQCHIyfJCiNRbFfDKdB8v6qchuwBSLXrEzhymeLp~81NeY3yPcivI0Ztps4T5TXrDxvLdIm68xsFl7jbJH5C~rUel0W2wwrTrvHOniDe8GoGJggttsSsf5uYxyYqhuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. T-cell priming following immunization with blood-borne antigen and adjuvant. (A) Images of in vivo antigen uptake by BM cells of a Cx3cr1gfp/+ mouse at different time points after injection of 0.5 mg fluorescently tagged OVA (yellow) and 10 nmol CpG. An hour after injection, the antigen had been taken up by many cell types, including green CX3CR1-GFP+ cells (arrows, insets, middle). After 24 hours, the fluorescent signal (arrows, right) persisted only in flat cells that line blood vessels (red), consistent with endothelial or perivascular cells (insets, right). Data are from 1 representative experiment of 2. Bar represents 50 µm. (B) Flow cytometric analysis of untreated BM or BM harvested 2 hours after intravenous injection of 0.5 mg fluorescently tagged OVA–Texas red with or without CpG. Histograms depict OVA acquisition, as indicated by the Texas red signal in pDCs (CD11cint mPDCA-1hi), T cells (CD3hi), B cells (CD19hi), conventional DCs (cDCs) (CD11chi MHC-II+), macrophages (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II+), Ly6Clo monocytes (CX3CR1-GFPhi, CD11cint, F4/80+, MHC-II−), and Ly6Chi monocytes (CX3CR1-GFPhi, CD11c−). Phagocytic capacity was slightly increased in CpG-treated mice, especially in the case of B cells. (C) Chimeric mice (CD11c-DTRtg×Cx3cr1gfp/+ into wild-type) were pretreated with DTx (right) or not (left) and then immunized with 0.5 mg OVA and 10 nmol CpG. Sixteen hours later, mice were transferred with GFP OT-I T cells (green) and CFP+ polyclonal CD8+ T cells (blue) to be imaged 4 hours later using 2PM. Regardless of DC depletion, OT-I cells formed antigen-dependent clusters. See also supplemental Video 10. Bar represents 50 µm. (D) Velocities of polyclonal and OT-I CD8+ T cells transferred into chimeric mice that had been injected with OVA + CpG and either treated or not with DTx as described in panel C. Data points represent individual cells. The mean velocity of polyclonal T cells and OT-I cells in untreated mice was 5.9 vs 2.8 μm/min (n = 51 and 54, respectively); in DTx-treated mice, the mean velocity was 6.4 vs 3.4 μm/min, (n = 63 and 74, respectively). Regardless of DC depletion, OT-I cells decelerated. Individual 2-tailed t tests compared OT-I and polyclonal T cells within each experimental condition. *P < .001. (E) Analysis of proliferative capacity following immunization with 0.5 mg OVA + CpG. The bars represent DI calculated from pooled data from 2 independent experiments involving 5 mice in each immunization condition. DC depletion significantly reduced proliferation in the spleen (DI of 0.6) but not in the BM (DI of 1.6). *P < .001. Two-way ANOVA was used to compare, within each organ, OVA + CpG–treated mice with the 2 other groups. Error bars represent SEM. (F) Images of GFP+ OT-I cells (green) transferred into OVA + CpG–injected [CD11c-DTRtg into wild-type] chimeric mice that had been left intact (left) or treated with DTx and CLL (right). T cells were analyzed by 2PM 48 hours after OVA challenge. T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were sparser and failed to divide. Insets depict a dividing cell. See also supplemental Video 11. Blood vessels were counterstained in red. Bar represents 50 µm. (G). Densities of OT-I cells in the BM, as calculated from several fields such as the ones depicted in panel F. T cells in the BM of OVA-injected mice depleted of all mononuclear phagocytes were significantly sparser. *P < .01. Error bars represent SEM. (H) In vivo killing ability of OT-I CTLs primed in the presence or absence of mononuclear phagocytes. 5000 Naïve OT-I -CFP T cells were transferred into CD11c-DTRtg into wild-type chimeric mice prior to treatment with DTx either with or without CLL. Mice were then immunized with 0.5 mg OVA and 10 nmol CpG. Eight days later, recipient mice were injected with equal numbers of CD45.1 CFSEhi splenocytes pulsed with OVA peptide and CFSElo unpulsed cells. Antigen-specific killing was tested in the BM and spleens of recipient mice 1 day later. Combined depletion, but not DC depletion alone, annihilated target cell killing in both the spleen and BM. Bars show normalized percentage specific killing. *P < .05; **P < .01; ***P < .001. A 2-way ANOVA was used to compare, within each organ, OVA + CpG–treated mice with the 3 other groups. Error bars represent SEM. cDCs, conventional DCs; SP, spleen.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/2/10.1182_blood-2012-01-401265/4/m_193f6.jpeg?Expires=1769317944&Signature=v~BcGuSqjk53rPG4FBXXi~oO7n48KMo2l~o57Xifr9mwOgq-ol5Q7cse~RVJvwk4ESPXwnIqtBnUqaF2fKy0Uq~eR0gfqhIb7kHuaoJO3l6jmEVuHoByMXVzZDGIm21wkRu2Mg6G0JZUIosyx5kbZ~E0D6CWi~30wHvID4osWWeNylNrf4V1oJfDJ1mZbuA6NM64QwDtzqGKnkgX5GEqNB6CJgntZdM3eFb2ORBPrsTUqBEiI4yX9ZSZwLnV-ab681okk~J72sZy7nT0x~FoIk~SCYNZI6X6CkfLgjk5RgSgNb46DniG8WIv5XZ64pJ4NVA4bPU1h8OWsUZ7fN-9qQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
