Abstract
Among the three classic Philadelphia-negative myeloproliferative neoplasms (Phneg MPN), myelofibrosis (MF) is the most severe in terms of survival and quality of life, with very limited therapeutic options.
Although the pathogenesis of Phneg MPN remains still poorly understood, aberrant megakaryocytopoiesis is a common, distinctive feature. Specifically, in MF, bone marrow megakaryocytes (MKs) are hyperplastic and show typical morphological abnormalities such as hypolobated nuclei, tendency to form tight clusters and impaired capacity to generate pro-platelets (pro-PLTs) in-vitro. Recent data proved that MF-MK hyperplasia is a consequence of both increased proliferation and reduced apoptosis of MK progenitors, likely correlated to the over-expression of the anti-apoptotic gene Bcl-xL (Ciurea et al. Blood 2007).
Protein Kinase Cε (PKCε) is a novel, calcium-independent PKC isoform, capable to modulate cell proliferation, differentiation and survival. Our group showed that PKCε plays a crucial role in normal and malignant hematopoiesis (Mirandola et al. Blood 2006; Gobbi et al. Stem Cells 2007; Gobbi et al. Blood 2009). During in-vitro erythroid and megakaryocytic differentiation of normal CD34+ progenitors, PKCε levels are finely tuned with a virtually opposite kinetic: progressively increasing during erythroid maturation while peaking early and then decreasing during MK maturation. Forced expression of PKCε in the later phases of megakaryocytopoiesis delays MK differentiation, proving that PKCε silencing is required for MK full differentiation.
Here we investigated the expression of PKCε in primary myelofibrosis (PMF)-MK progenitors and we tested whether PKCε modulation may affect megakaryocytic differentiation of PMF-CD34+ cells.
CD34+ cells were immunomagnetically isolated from 5 PMF patients and 2 G-CSF mobilized donors (controls, C) and then cultured up to 14 days in serum-free medium supplemented with 200 ng/mL thrombopoietin, 50 ng/mL Stem Cell Factor and 3 ng/mL Interleukin-3. MK differentiation was assessed by morphological analysis and in-vitro pro-PLT generation.
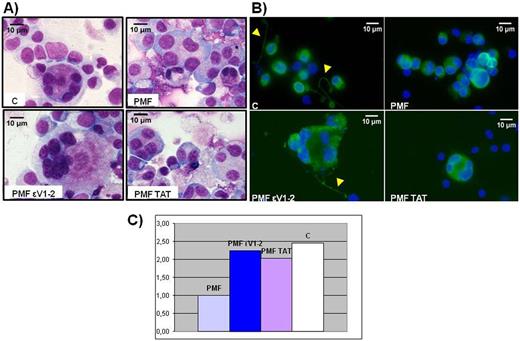
Consistently with current literature, also in our serum-free based culture, PMF-MKs showed impaired differentiation associated with abnormal morphology (smaller size, round and hypolobated nuclei) and reduced pro-PLT generation when compared to C (Fig. 1 panel A).
First, we demonstrated by Western Blot analysis (WB), that PMF-CD34+ displayed higher levels of PCKε, phosphorylated PKCε (pPKCε), Bcl-xL and Bcl-2 (Fig. 1, panel B ).
This is consistent with their augmented proliferative capacity in MK-differentiating medium [median fold increase of PMF cultures was significantly higher than C (12.91 vs 1.09, respectively, p<0.05)].
Additionally, during in-vitro MK differentiation, PKCε levels of PMF-MKs were significantly higher than C-MKs at any time point of the culture analyzed by WB (a representative picture is shown in Fig. 1, panel C).
Consequently, we sought to determine whether PKCε inhibition was able to restore a normal in-vitro MK maturation assessed by: i) MK morphology ii) pro-PLT formation, iii) number of PLTs released in the culture medium (evaluated as number of CD41+/calcein AM+ cells, as described in Gobbi et al. Blood 2013).
PKCε activity was pharmacologically modulated in two different experiments by the εV1-2 (CEAVSLKPT) peptide conjugated to TAT47-57 (CYGRKKRRQRRR) by a cysteine disulfide bound (Brandman J. Biol. Chem. 2007).
As shown in Fig. 2, treatment with εV1-2 was capable to restore a normal MK morphology (panel A) and adequate pro-PLT formation (panel B). Addition of the sole vehicle (TAT47-57) in the culture medium did not provide any improvement on cell maturation, proving that the effects we observed were entirely attributable to PKCε-inhibition by εV1-2.
Additionally, a clear trend in terms of increase of the number of PLTs released in the media of PMF cultures treated with εV1-2 was shown (Fig. 2, panel C).
Our data demonstrate for the first time a potential involvement of PKCε in the pathogenesis of MF and that PKCε inhibition may revert, in-vitro, the abnormal megakaryocytopoiesis that typifies this neoplasm. Since PKC and PKCε are currently under investigational use in a number of diseases, PKCε inhibition may configure as a new potential therapeutic strategy for MF patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.