Abstract
Adult T-cell leukemia/lymphoma (ATLL) is a rare aggressive malignancy with a poor prognosis caused by HTLV-1. Miami is proximal to the Caribbean where HTLV-I is endemic, and we encounter a relatively high number of ATLL cases. Herein, we have performed the largest single institution retrospective analysis of ATLL patients (pts) to date in the U.S. We studied ATLL patient characteristics, treatment patterns, and disease outcome. In addition, we investigated the expression of IRF-4/MUM-1 in available specimens. Previously, our group demonstrated an association between lack of IRF-4/MUM-1 and response to AZT-interferon-alpha (AZT/IFNa) therapy in a small ATLL cohort. IRF-4/MUM-1 is a putative NF-kB target gene that encodes a transcription factor. IRF-4/MUM-1 expression has been associated with interferon resistance in preclinical studies, and is a poor prognostic marker in some lymphomas. One of our objectives is evaluate and validate IRF-4/MUM-1 as a potential biomarker for treatment selection and outcome in ATLL.
We analyzed 125 pts diagnosed with ATLL in UM/JMH between 1987 and 2013. We evaluated MUM-1 protein expression using immunohistochemistry (IHC) on either tissue sections, or cytospins prepared from CD4+-enriched peripheral blood leukemic specimens using 30% nuclear staining as a cut-off positive value, or by western blot (WB) analysis in some cases where fresh or DMSO preserved ATLL cells were not available. Kaplan-Meier survival curves, log-rank test where used for survival analysis. Mann-Whitney's U test was used to compare non-normally distributed continuous variables. Pearson's chi-squared or Fischer's exact tests were used to compare categorical variables.
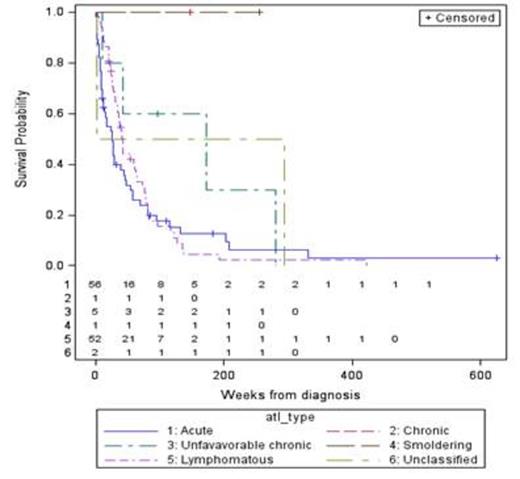
ATLL pts were 45% male and 55% female with a median age of 51 (17-91). The great majority of pts were Afro-Caribbean (82%), followed by U.S. African American (12%) and South American (6%). A total of 109 pts have been analyzed for treatment response so far, including 51 acute, 50 lymphomatous (L), 6 chronic (5 unfavorable), and 2 smoldering types. The median overall survival (OS) for acute and L was 6 and 10 months respectively, and not reached for chronic and smoldering types (figure 1). Fifty-six pts (34 acute, 14 L, 5 unfavorable chronic, 1 chronic, and 2 smoldering) were treated with high-dose AZT/IFNa as first line therapy. The complete and overall response rates (CR and ORR) after AZT/IFNa for acute/unfavorable chronic (A/UC) vs. L types were 25% vs. 0.7%, and 54% vs. 21% respectively. Seventy-seven pts received chemotherapy at some point during their treatment. The CR rate and ORR for A/UC vs. L-type pts treated with chemotherapy-based regimens were 40 % vs. 21%, and 70% vs. 77% respectively. However, we observed a significantly longer median progression-free survival (PFS) and sustained responses in pts with A/UC ATLL who achieved a CR with AZT/IFNa (168.1 wks), as compared to chemotherapy (61.1 wks), which translated into an overall survival benefit.
OS according to ATLL subtype
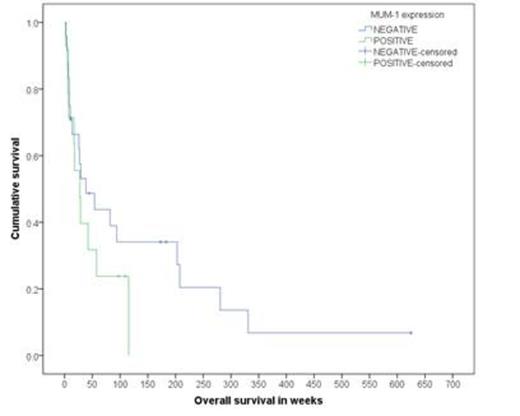
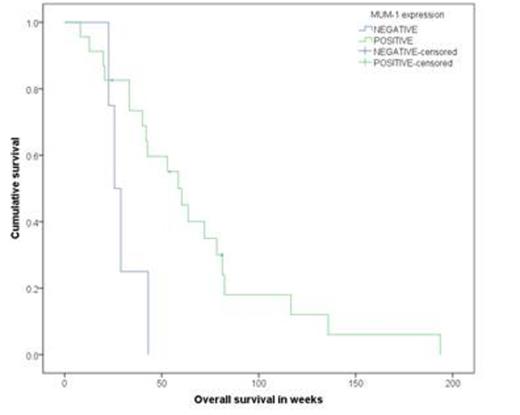
Next, in order to determine whether IRF-4/MUM-1 predicted response to AZT/IFNa, 66 ATLL cases were analyzed by IHC and/or WB. The results showed that 38.5% of A/UC were IRF-4/MUM-1+ as compared to 82.1% in the L type (<P.0001). Evaluable pts for AZT/IFNa response demonstrated that 55% of A/UC MUM-1(-) cases had a CR as compared to 0% in MUM-1+ pts (P=.009). In the L group, AZT/IFNa responses were minimal and were mainly limited to stable disease. Subgroup analysis showed that median OS for MUM-1(-) vs. MUM-1+ in A/UC subtype was 38.4 wks vs. 27.6 wks (P=0.275) (figure 2), respectively. In the L subtype, median OS for MUM-1(-) vs. MUM-1+ was 25.7 wks vs. 60.3 wks (P=0.02) (figure 3), respectively. Finally, the median PFS in AZT/IFNa-treated A/UC pts favored the MUM-1(-) as compared to MUM-1+ cases.
OS in acute/unfavorable chronic type by MUM-1 status
OS in lymphomatous type by MUM-1 status
Our data demonstrate that AZT/IFNa therapy is beneficial in leukemic type (A/UC) lacking IRF-4/MUM-1 expression, while L type is generally resistant to this treatment. On the other hand, IRF-4/MUM-1 expression is associated with a favorable outcome in L subtype. We have identified IRF-4/MUM-1 expression as a predictive marker that could be used in deciding upfront therapy (i.e. AZT/IFNa vs. standard chemotherapy) in leukemic ATLL subtypes. Our study findings must be confirmed and validated in a larger ATLL cohort.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.