Abstract
CML patients (pts) enjoy prolonged leukaemia-free survival with tyrosine kinase inhibitor (TKI) treatment. Addressing common non-CML causes of morbidity and mortality such as cardiovascular disease (CVD) and its associated risk factors is therefore increasingly important. Anecdotal evidence suggests a possible association between nilotinib (NIL) therapy and vascular events though there is little good quality evidence in this regard. The incidence of hyperlipidaemia and hyperglycaemia is higher in NIL-treated pts compared to imatinib- (IM) treated pts; conversely IM treatment may retard development of dyslipidaemia and hyperglycaemia. This retrospective analysis assessed the lipid profile of CML-CP pts before and after changing from IM to NIL therapy.
Plasma lipid profile (total cholesterol, LDL, HDL and triglycerides), TKI exposure, and CVD risk factors before and after switchover to NIL of chronic phase CML pts were analysed. Baseline measurements were done during IM treatment or within 2 weeks of changing from IM to NIL. Follow-up results were obtained at least 1 month after starting NIL.
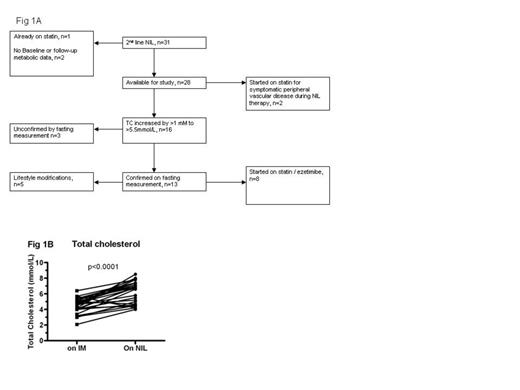
Thirty-one CML pts were switched to NIL (median dose 400mg bd) after a median of 27 (1-96) months of IM therapy, predominantly for intolerance (16/31, 52%) or for failure to achieve deep molecular responses (13/31, 42%).
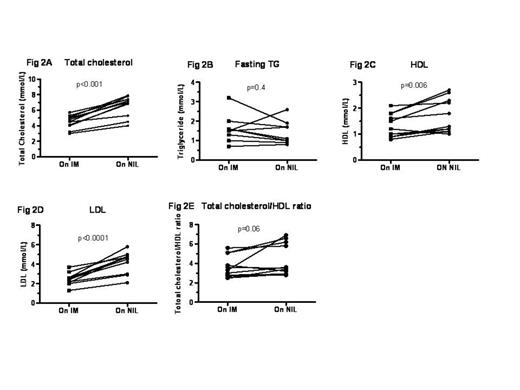
Median age at NIL start was 51 (17-80) years (Table I). After switching to NIL, three pts had new onset PVD/IHD and one patient had multiple recurrences of PVD. Antihypertensive and hypoglycaemic medications were started in one additional patient each after switching over to NIL. Three pts were excluded from further analysis because of lack of data (n=2) or pre-existing dyslipidemia (n=1). The remaining pts had 90 TC assays, 72 of which were full lipid profiles whilst on NIL. Observations were censored at the time of statin commencement. Median time to 1st lipid measurement on NIL was 108 days (28-633) after switching. Median TC on IM was 4.7mM (2.1-6.4), compared to 6.1mM on NIL (3.1-8.5). Median peak TC on NIL was 6.8mM. Full fasting lipid profiles available for 11 pts before and after switching showed significantly increased LDL to be the major contributor to the increase in TC (Fig. 2).
Total cholesterol level was significantly higher after switching to NIL therapy. (A) Schematic of pts in this study (B) TC level during IM and NIL therapy
Total cholesterol level was significantly higher after switching to NIL therapy. (A) Schematic of pts in this study (B) TC level during IM and NIL therapy
Characteristics of pts before and after switching over to nilotinib
| Characteristics . | On Imatinib n=31 . | On Nilotinib n=31 . |
|---|---|---|
| Median follow up (range) | 27 (1-96) | 31 (7-93) |
| Median age (range) | 48 (16-79) | 51 (17-79) |
| Intolerant to imatinib | 16/31 (52%) | |
| Median total cholesterol mmol/L (range) | 4.7 (2.1-6.4) | 6.8 (4-8.5) |
| Hypoglycaemic treatment at anytime | 2 (6%) | 3 (10%) |
| Anti-Hypertensive treatment at any time | 4 (13%) | 5 (16%) |
| Symptomatic peripheral vascular disease or ischemic heart disease | 1 (3%) | 4 (13%) |
| Dyslipidemia requiring statins | 1 (3%) | 11 (35%) |
| Characteristics . | On Imatinib n=31 . | On Nilotinib n=31 . |
|---|---|---|
| Median follow up (range) | 27 (1-96) | 31 (7-93) |
| Median age (range) | 48 (16-79) | 51 (17-79) |
| Intolerant to imatinib | 16/31 (52%) | |
| Median total cholesterol mmol/L (range) | 4.7 (2.1-6.4) | 6.8 (4-8.5) |
| Hypoglycaemic treatment at anytime | 2 (6%) | 3 (10%) |
| Anti-Hypertensive treatment at any time | 4 (13%) | 5 (16%) |
| Symptomatic peripheral vascular disease or ischemic heart disease | 1 (3%) | 4 (13%) |
| Dyslipidemia requiring statins | 1 (3%) | 11 (35%) |
Fasting lipid profile in 11 CML pts whilst on IM and after switching to NIL therapy. Total cholesterol, HDL, and LDL levels increased significantly after switching to NIL. There was no significant change in triglycerides.
Fasting lipid profile in 11 CML pts whilst on IM and after switching to NIL therapy. Total cholesterol, HDL, and LDL levels increased significantly after switching to NIL. There was no significant change in triglycerides.
Thirteen pts had a fasting TC >5.5 mM (210 mg/dL) whilst on NIL, peaking at 312 days (medians). Eight of 13 pts started statins treatment for dyslipidemia whilst on NIL; (all retrospectively confirmed to be appropriate according to Australian National Heart Foundation guidelines, using a composite measure of CVD risk); whilst the other 5 were offered lifestyle modifications only. Of note, 2 pts had dyslipidaemia prior to starting IM treatment, discontinued statins whilst on IM, and had recurrence of dyslipidaemia after switching to NIL necessitating resumption of statin treatment. In addition, the 2 pts with PVD were also offered statins (total starting statin n=10)
This retrospective analysis showed a high incidence of dyslipidemia in a cohort of CML pts treated with second-line NIL after IM therapy; 10/31 pts required lipid lowering agent. While the epidemiological association between nilotinib and CVD remains controversial, the increase in TC may have a contributing effect. Monitoring lipid levels in NIL-treated pts is prudent, along with screening for and minimisation of concomitant CVD risk factors, especially in pts previous treated with IM which may mask underlying metabolic syndromes.
# DKH DTY and LC contributed equally to this work.
Hiwase:Novartis Pharmaceuticals: Research Funding. Yeung:Novartis: Honoraria, Research Funding; BMS: Honoraria. Hughes:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Ariad: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.