Abstract
Analysis for genomic alterations by fluorescence in-situ hybridization (FISH) allows prognostic stratification in chronic lymphocytic leukemia (CLL). Recurrent mutations in genes including NOTCH1, SF3B1, BIRC3 and others have recently been identified in patients with CLL by next-generation sequencing and may provide independent prognostic information (Rossi D et al. Blood 2013). The prognostic impact of these mutations following first-line therapy chemotherapy has not been consistent in the literature (Oscier D et al. Blood 2012; Ysebaert L et al. ASH Abstract 2012; Stilgenbauer S et al. ASH Abstracts 2012) and the prognostic significance of these mutations in elderly patients requiring therapy is not clearly defined (Chiaretti S et al. ASH Abstracts 2012).
We aimed to describe the incidence of genomic alterations by FISH and multiplexed targeted resequencing of genes with potential prognostic importance in an elderly population of patients requiring frontline treatment for CLL.
Pre-treatment peripheral blood and bone marrow aspirate samples were obtained from patients enrolled on a phase II randomized clinical trial investigating oral fludarabine, oral cyclophosphamide and i.v. rituximab (poFCivR) tolerance in previously untreated fit elderly patients with CLL (Australasian Leukemia and Lymphoma Group [ALLG] CLL5, ACTRN12608000404325). Peripheral blood lymphocytes were purified by Ficoll gradient separation. Bone marrow aspirate samples were analyzed for CLL-associated genomic changes with a Vysis CLL FISH probe kit (Abbott, Des Moines, IL). DNA was extracted from peripheral blood samples and we performed targeted genome sequencing of genes including TP53, ATM, NOTCH1, SF3B1, BIRC3, MYD88, XPO1 and FBXW7 using a TruSeq Custom Amplicon Design Panel on a MiSeq Sequencer as per manufacturer's protocol (Illumina, San Diego, CA). DNA mutation data was analyzed and compared to reported mutations in the literature and COSMIC database. Non-synonymous mutations with a mutation frequency >10% are reported.
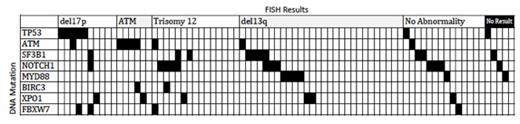
120 patients were enrolled into the CLL5 study and 78 samples were available for analysis. In these patients, FISH analysis identified 17p deletion in 10 pts (13%), 11q deletion in 6 pts (8%), trisomy 12 in 15 pts (19%), deletion 13q in 28 pts (36%), no abnormality (n=14, 18%), and not performed (n=5, 6%). By targeted sequencing, mutations were identified in TP53 (n=7, 9%), ATM (n=7, 9%), NOTCH1 (n=12, 15%), SF3B1 (n=10, 13%), MYD88 (n=6, 8%), BIRC3 (n=2, 3%), XPO1 (n=6, 8%), FBXW7 (n=4, 5%). Thirty patients (38%) did not have a mutation in one of these genes. Mutation distribution is demonstrated in Figure 1. Five of 7 mutations in TP53 occurred in the DNA-binding core domains (exons 5-8) and one mutation in the homo-oligomerization domain and one in exon 11. The majority of NOTCH1 mutations were identified in the PEST domains (11 of 12) and all but one mutation in SF3B1 were identified in the PP2A repeats 5-9. Correlation with patient characteristics, IGHV mutational sequencing and clinical outcome data is proceeding.
Figure 1.
We demonstrate a high proportion of genomic mutations in fit patients age 65 years and older undergoing first-line therapy for CLL. By comparison with data from Italian CLL trials, UK LRF CLL4 and the CLL8 study, our study demonstrates similar mutational frequencies in previously described recurrently mutated genes. These mutations are assessable by massive parallel sequencing and may potentially offer useful predictive information in patients requiring therapy for CLL.
Supported by grants from CLL Global Research Foundation and Cancer Institute of NSW / Northern Translational Cancer Research Unit.
Mulligan:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.