Abstract
Systemic anaplastic large cell lymphoma (sALCL) is a CD30-positive aggressive subtype of mature T-cell lymphoma. Approximately 40–65% of patients (pts) with sALCL develop recurrent disease after frontline treatment. Outcomes are poor for pts with relapsed T cell lymphomas, including sALCL, with a median overall survival (OS) of 7.0 mos (Mak et al, 2013). Few effective therapies are available to address this unmet need. Brentuximab vedotin (ADCETRIS®) is an antibody-drug conjugate comprising a CD30-directed antibody attached to the microtubule-disrupting agent monomethyl auristatin E (MMAE) via a protease-cleavable linker. A phase 2 study evaluated the efficacy and safety of brentuximab vedotin in 58 pts with relapsed or refractory sALCL (ClinicalTrials.gov #NCT00866047). Long-term follow-up data from this ongoing trial are presented.
Pts received 1.8 mg/kg brentuximab vedotin every 3 weeks as a 30-minute outpatient IV infusion for up to 16 cycles. The primary endpoint was the objective response rate (ORR) per independent review according to the Revised Response Criteria for Malignant Lymphoma (Cheson 2007).
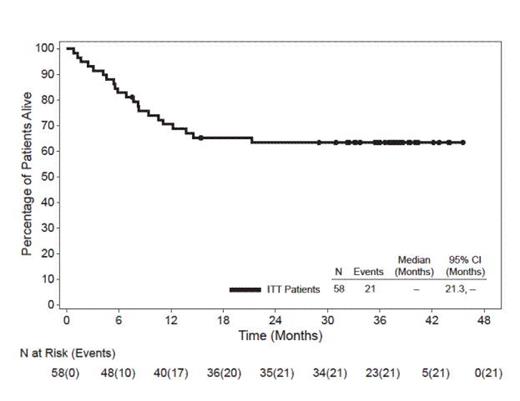
In this heavily pre-treated pt population with poor prognosis, 62% had primary refractory disease (defined as no complete remission [CR] or relapse within 3 mos of frontline therapy), 26% had failed a prior autologous stem cell transplant (SCT), and 72% had ALK-negative disease. As previously reported, the ORR with brentuximab vedotin was 86% (50 of 58 pts) and the CR rate was 59% (34 of 58 pts). At the time of this analysis (datacut June 2013), all pts had discontinued treatment and the median observation time from first dose was 33.4 mos (range, 0.8-45.6). The median duration of objective response for all pts was 13.2 mos (95% CI: 5.7, 26.3) and the median duration of response for pts who obtained a CR was 26.3 mos (95% CI: 13.2, -). Of the 34 pts who achieved a CR, 16 (47%) remained in remission at the time of this analysis. Thirty-seven of 58 pts (64%) were alive at the time of last follow up. The median progression-free survival (PFS) for all pts was 14.6 mos and the median OS has not yet been reached. The estimated 3-year survival rate was 63% (95% CI: 51%, 76%). Median OS for pts who obtained a CR has not yet been reached while median OS for pts who did not obtain a CR was 7.7 mos (95% CI: 4.5, 13.7). Median OS for pts with PET-negative disease at Cycle 4 has not yet been reached while median OS for pts with PET-positive disease at Cycle 4 was 14.6 mos. After discontinuing treatment in the study, 17 pts (29%) received a hematopoietic SCT (9 allogeneic, 8 autologous). The median PFS has not yet been met for the group of pts who achieved a CR and received a subsequent SCT (95% CI: 14.6, -), while the median PFS for the group who achieved a CR and did not receive post-treatment SCT was 18.4 mos (95% CI: 8.4, 33.7). As previously reported, adverse events (AEs) in ≥20% of pts were peripheral sensory neuropathy (41%), nausea (40%), fatigue (38%), pyrexia (34%), diarrhea (29%), rash (24%), constipation (22%), and neutropenia (21%). The majority of AEs were Grade 1 or 2 in severity.
After a median observation time of 33.4 mos from first dose of brentuximab vedotin, 64% of pts with relapsed or refractory sALCL were alive at the time of last follow up and the median OS has not yet been reached. Pts who achieved a CR with brentuximab vedotin experienced longer OS than pts who did not achieve a CR and early PET-negative status appeared to be important for long-term survival. These long-term follow-up results further underscore the durability of clinical benefit obtained with brentuximab vedotin. A randomized phase 3 study is being conducted to evaluate brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone for frontline treatment of CD30-positive mature T-cell lymphomas, including sALCL (ClinicalTrials.gov #NCT01777152).
Overall Survival
Pro:Seattle Genetics, Inc.: Advisory/Scientific board membership and travel expenses Other, Consultancy, Research Funding. Advani:Seattle Genetics, Inc.: Advisory/Scientific Board Membership Other, Research Funding. Brice:Seattle Genetics, Inc.: Honoraria, Research Funding. Bartlett:Seattle Genetics, Inc.: Advisory/Scientific Board Membership and Travel Expenses Other, Research Funding. Rosenblatt:Seattle Genetics, Inc.: Research Funding. Illidge:Seattle Genetics, Inc.: Consultancy, Research Funding. Matous:Seattle Genetics, Inc.: Research Funding, Speakers Bureau. Ramchandren:Seattle Genetics, Inc.: Research Funding, Speakers Bureau. Fanale:Seattle Genetics, Inc.: Advisory/Scientific Board Membership and Travel Expenses Other, Consultancy, Honoraria, Research Funding. Connors:F Hoffmann-La Roche: Research Funding; Roche Canada: Research Funding. Yang:Seattle Genetics, Inc.: Employment, Equity Ownership. Huebner:Takeda: Equity Ownership; Takeda Cambridge US: Employment. Kennedy:Seattle Genetics, Inc.: Employment, Equity Ownership. Shustov:Seattle Genetics, Inc.: Advisory/Scientific Board Membership Other, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.