Abstract
MGUS and Multiple Myeloma (MM) are two to three times more common in African Americans (AA) compared to Caucasians (CAU). They also differ in many disease characteristics, most notably the better disease specific survival of AA compared to CAU. Recent improvements in outcomes have helped the CAU population, not benefitting the AA to the same degree. Although this disparity is well appreciated, little is know regarding the associated molecular differences.
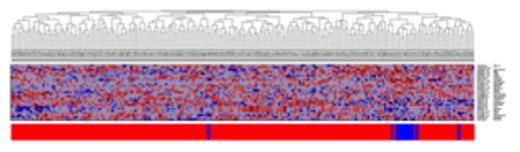
Heatmap for 27-gene model, training set
(Key: blue = African-American, red=Caucasian)
Heatmap for 27-gene model, training set
(Key: blue = African-American, red=Caucasian)
Heatmap for 27-gene model, test set
(Key: blue = African-American, red=Caucasian)
Heatmap for 27-gene model, test set
(Key: blue = African-American, red=Caucasian)
Heatmap, 27-gene model, AMM validation set
(Key: blue = African-American, red=Caucasian)
Validation set: 63 Caucasian, 7 African-American with AMM baseline GEP samples
Heatmap, 27-gene model, AMM validation set
(Key: blue = African-American, red=Caucasian)
Validation set: 63 Caucasian, 7 African-American with AMM baseline GEP samples
Summary measures of the 27-gene model, test set
| Measure . | Value . |
|---|---|
| Sensitivity | 9/13 (69.2%) |
| Specificity | 205/209 (98.1%) |
| Positive Predictive Value | 214/222 (96.4%) |
| Measure . | Value . |
|---|---|
| Sensitivity | 9/13 (69.2%) |
| Specificity | 205/209 (98.1%) |
| Positive Predictive Value | 214/222 (96.4%) |
Summary measures of the 27-gene model, AMM validation set
| Measure . | Value . |
|---|---|
| Sensitivity | 5/7 (71.4%) |
| Specificity | 62/63 (98.4%) |
| Positive Predictive Value | 67/70 (95.7%) |
| Measure . | Value . |
|---|---|
| Sensitivity | 5/7 (71.4%) |
| Specificity | 62/63 (98.4%) |
| Positive Predictive Value | 67/70 (95.7%) |
The bone marrow microenvironment (ME) has a significant impact on the behavior of myeloma cells. We therefore also sought to investigate the differences in the bone marrow ME between AA and CAU. Using a set of 783 probesets that are unique to the bone marrow microenvironment, we analyzed GEPs of whole bone marrow biopsies in a training set of 250 CAU and 22 AA patients, identifying only 7 probesets with a false discovery rate <0.1. A score created based on these 7 genes had a sensitivity of only 66.7%, a specificity of 76.5% and a positive predictive value of 76.1%.
In summary we identified a list of genes, which are differentially expressed between AA and CAU and have developed a model that can accurately identify race based on the expression of 27 genes. With the data available we were not able to develop a suitable model that identified differences of the bone marrow ME between AA and CAU. Correlation of the gene expression differences of plasma cells with clinical characteristics and outcome will provide us with a better understanding of the pathophysiology of MM in AA and will hopefully be the basis of individualized therapy that also takes ethnicity into consideration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.