Abstract
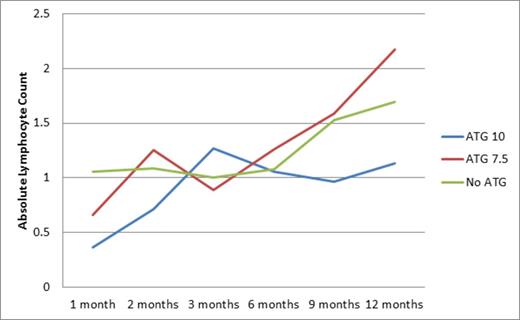
Anti-thymocyte globulin (ATG) is widely used for in vivo T cell depletion and immunomodulation in unrelated donor (URD) stem cell transplantation (SCT) to reduce the risk of graft vs. host disease (GVHD). However, despite the reduction in GVHD risk, outcomes are generally not superior to matched related donor (MRD) SCT conditioned without ATG. This is primarily because of defective immune reconstitution and high rates of opportunistic infections in ATG recipients. We have previously reported equivalent outcomes in URD SCT recipients conditioned with ATG when compared to MRD recipients. We now report immune reconstitution in an expanded cohort of these patients. Patients with AML, ALL, MDS, MPD (n=142) transplanted between 2004 and 2011 were included in this retrospective review. Seventy eight received either bone marrow or peripheral blood stem cell (PBSC) grafts from URD and received either 10 or 7.5 mg/kg rabbit ATG (Thymoglobulin, Sanofi-Aventis) during conditioning, those with MRD did not. Conditioning was myeloablative in all patients. Lymphoid recovery was equivalent in the two cohorts during the first year following SCT except in the first month (Figure), when URD recipients had lower absolute lymphocyte count (μ-URD=0.6x103/ μ L, μ-MRD=1.1; repeated measures mixed model p=0.022). Age, CD3/34 cell dose infused, stem cell source and conditioning intensity were not associated with ALC recovery post transplant. In a subset of patients lymphocyte subset enumeration was performed following withdrawal of immunosuppression, at an average 237 days post-SCT. ATG recipients had significantly lower mean CD4+ counts (μ-URD=267 (n=30), μ-MRD =434/ μ L (n=27); ANCOVA p = 0.003), however no significant differences were observed in CD3+, CD8+, CD19+ or CD56+ cell recovery. ATG recipients were significantly more likely to have complete donor T cell chimerism at 1 (OR = 12.5, CI= 2.4, 66.0, p = 0.001) and 2 months post-SCT (OR = 6.5 , CI=1.5, 27.4, p = 0.013), however by 9 months following SCT this trend had reversed with a greater likelihood of mixed T cell chimerism (OR > 100; p = 0.017), suggesting re-emergence of recipient derived T cell clones. Consequently, donor lymphocyte infusions were given significantly more often to ATG recipients (12/78) than to non-recipients (2/64) (OR = 5.64, CI = 1.21, 26.20, p=0.027). High grade CMV viremia (1000 copies/ μL) was significantly more likely in CMV sero-positive ATG recipients (n=18/55) than in non-recipients (n=7/48) (OR = 2.8, CI 1.1, 7.6, p = 0.032). Reduced intensity conditioning and PBSC were associated with higher CMV reactivation in ATG recipients and there was a lower likelihood of survival in these individuals than in those who did not receive ATG (HR: 0.53, CI: 0.31, 0.92; p = 0.024). EBV reactivation was observed more often in susceptible ATG recipients (n=22/58) than in non-recipients (n=5/43), (OR=4.6, CI=1.6, 13.6, p= 0.003). The median peak EBV viral load in ATG recipients (u=1545 copies/ μL, IQR: 288, 2,302) was significantly higher than in non-recipients (u=120 IQR: 57, 169, p = 0.005). PBSC stem cell source (p = 0.049) and HLA mismatch (p =< 0.001) were associated with EBV reactivation in ATG recipients but not in non-recipients. ATG recipients were also more likely to experience a fungal infection (OR=2.8, CI=1.1, 6.7, p=0.023). 1-month ALC was predictive of disease free survival whereby it had a significant negative effect on relapse (HR = 0.33; 95% CI: 0.16, 0.66; p = 0.002). As 1-month ALC increased by one-tenth, the odds of relapse decreased by over 3% and survival increased by 3%. In conclusion, high doses of ATG used during conditioning are associated with an early retardation of lymphoid recovery post-SCT, and with late mixed T cell chimerism accompanied by a delay in CD4+ T cell recovery. This is associated with a higher rate of viral reactivation in PBSC recipients and of fungal infections in general. Lower doses of ATG should be used in SCT and in ATG conditioned SCT, early intervention with DLI, particularly CD8+ cell depleted DLI as reported by others, may help restore T cell repertoire and improve SCT outcomes and survival.
Absolute Lymphocyte Counts over time.
Toor:Sanofi Avnetis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.