Abstract
X-linked Sideroblastic Anemia (XLSA, MIM# 300751) is due to mutations in the erythroid-specific form of 5-aminolevulinate synthase (ALAS2) gene. Main features of this condition are microcytic anemia, iron deposits in the mitochondria of erythroid precursors (ring sideroblasts) and an X linked pattern of inheritance. However, up to one third of described cases have been reported in females mainly due to a highly skewed X-chromosome inactivation (Ducamp, Kannengiesser et al. 2011). We report for the first time in a large four generations pedigree a new mutation in ALAS2 gene inducing a Male Lethal X-linked Syndrome ascertained through an adult heterozygous female with a mild form of congenital sideroblastic anemia (CSA).
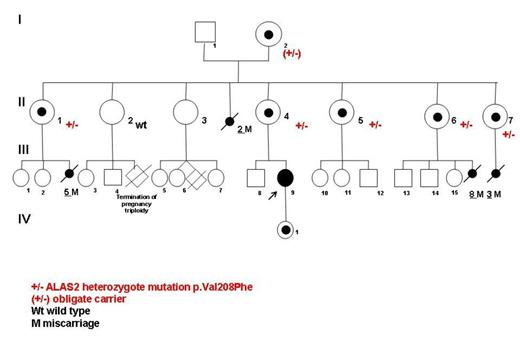
The propositus of this non consanguineous family (Fig 1; individual III;9) was a female from European ancestry. She exhibited a unexplained congenital, non regenerative, macrocytic (MCV 107fL, moderate anemia (Hb 10.4 g/dL), (first assessment at 6 years old). RBC transfusions were required only twice during pregnancy. The diagnosis of CSA was made at 23 years old when the bone marrow aspiration performed, showed 38% of ring sideroblasts. Erythrocyte protoporphyrin concentration was measured in the female proband carrying an ALAS2 mutation. The protoporphyrin concentration was within the normal range of values: 1.6 µmoles/L of red blood cells (less than 1.9 µmoles/L of red blood cells), as previously observed in XLSA cases. The level of serum ferritin was 224ng/ml (N:11-306) and transferrin saturation was 90%. A heterozygote ALAS2 deleterious missense mutation c.622G>T,p.Val208Phe affecting a conserved amino acid was found. A constitutive skewed X-chromosome inactivation was demonstrated as previously reported in affected females with XLSA. However erythroid bone marrow precursor did not exhibited different pattern repartition in term of apoptosis or dyserythropoisesis. Her daughter and her mother exhibited the same mutation but did not have skewed X-chromosome inactivation and were unaffected with a normal blood count. A close inspection of the pedigree confirms a large female predominance (22 females/ 7 males) over four generations (/F/M ratio 3.1). No affected male were identified in the pedigree. Moreover a high level of miscarriage was found only in female carrying the ALAS2 mutation, as shown in the pedigree (Fig. 1). Adding the number of miscarriage (18 over the four generations) to the number of males alive the ratio of M/F over 4 generation is close of 1: 1.04 (24/23). These data highly suggest an X-linked dominant disorder with pre natal male lethality.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.