Abstract

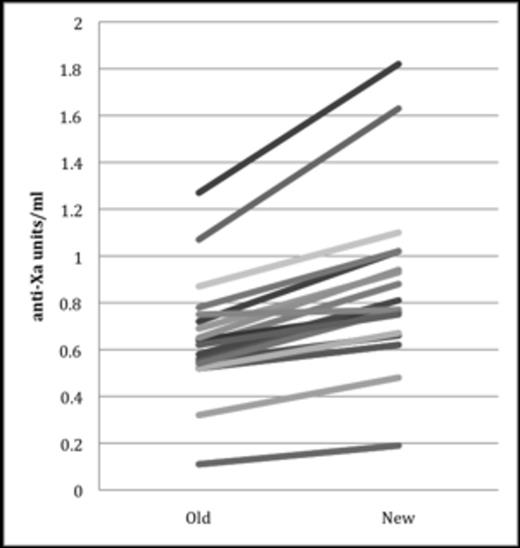
Due to favorable pharmacokinetic properties, enoxaparin is the most frequently used anticoagulant in children. Unlike adults, a developing hemostatic system provides rationale for therapeutic monitoring with an anti-Xa goal of 0.5-1 units/ml. However, accepted therapeutic ranges are not well correlated with clinical outcomes (i.e. thrombosis or hemorrhage) and there is no assay standardization. In 2011 the coagulation laboratory at the Children's Hospital of Philadelphia (CHOP) changed anti-Xa laboratory assays resulting in anti-Xa levels that were on average, 33% higher than the prior assay (Figure 1). We describe how this change in assay influenced therapeutic enoxaparin dose (mg/kg) at our center.
The coagulation laboratory performed simultaneous anti-Xa measurements using the Stachrom (old) assay and Rotachrom (new) assay on 19 subjects from split plasma samples. The new assay was then implemented into clinical practice. Information on anti-Xa values and enoxaparin dosing during the initiation of anticoagulation was retrospectively compiled for the 6 months pre and post assay change. Data on enoxaparin dosing and anti-Xa values was collected on 109 children in the old assay phase and 104 children in the new assay phase. Therapeutic enoxaparin dose was defined as the first dose resulting in an anti-Xa value 0.5- 1 unit/ml. All subjects initiated on anticoagulation during this 12 month period were included. Anti-Xa levels were obtained per CHOP standard clinical monitoring: until therapeutic, after dose adjustment, and every other week while inpatient with some subjects more frequently evaluated per provider discretion. Cumulative number of inpatient anti-Xa values obtained was determined for each subject. Enoxaparin dosing and cumulative anti-Xa values pre and post assay changes in each cohort were analyzed using a t-test.
Using 19 split samples there was strong correlation between the two assays (r=0.96), however, the means between the two assays differed significantly (p <0.0001) with new assay findings being, on average, 33% higher (Figure 1). There was a significant decrease in the new assay mean therapeutic enoxaparin dose (mg/kg) in subjects <3 months (p=0.01) and 3 months–2 years (p<0.0001), but not in subjects >2 years (p=0.18) (Table 1). However, the mean first therapeutic anti-Xa value was significantly higher in subjects > 2 yrs post-assay change (p=0.001). Among all 213 subjects, a total of 1,061 inpatient anti-Xa values were obtained, with a median of 3 (range: 1-32) per subject. Median number of anti-Xa values obtained to achieve therapeutic range was 2 (range: 1-6) prior to assay change and 1 (range: 1-7) post assay change with a significant difference (p=0.004) observed between the groups.
Anti-Xa values determined by Stachrom and Rotachrom assays on split plasma samples
Anti-Xa values determined by Stachrom and Rotachrom assays on split plasma samples
Comparison of mean enoxaparin therapeutic dose and first therapeutic anti-Xa value in patients using the Stachrom (old) assay to the Rotachrom (new) assay
 |
 |
S.D.= standard deviation
Using split patient samples, the Rotachrom assay demonstrated significantly higher anti-Xa values than the Stachrom assay. Once the Rotachrom assay was clinically implemented, there was a significant difference in weight based enoxaparin dosing in subjects < 2 years, and higher anti-Xa levels in subjects > 2 yrs. These observations demonstrate enoxaparin dose titration to achieve therapeutic anti-Xa is vulnerable to assay variability. Since these assays are not well standardized, this practice is likely to provide false reassurance of efficacy and safety. The large number of anti-Xa levels obtained may represent unnecessary venipuncture as well as misappropriation of time and resources. It is possible the majority of neonates and children may be safely and effectively treated with weight based dosing without monitoring. These data support a randomized controlled clinical trial in neonates and children comparing safety and efficacy of enoxaparin weight based dosing to dosing titrated to achieve therapeutic anti-Xa.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract