Abstract
We have recently reported that hematopoietic stem/progenitor cells (HSPCs) that harbor mutations of the PIG-A gene are preferentially mobilized into peripheral blood (PB) during hemolytic events in paroxysmal nocturnal hemoglobinuria (PNH) patients (Leukemia 2012;26:1722). This effect has been explained by i) an increase in the plasma level of sphingosine-1-phosphate (S1P), which at physiological doses is a major chemoattractant for HSPCs and is released from lysed erythrocytes (Leukemia 2010;24:976), and ii) the fact that PNH-cloned HSPCs, in contrast to normal HSPCs, show defective SDF-1–CXCR4-mediated retention in BM niches. It is known that under steady-state conditions the concentration of S1P in PB is already 25x higher than its concentration in the BM microenvironment and increases additionally during hemolysis.
Since erythrocytes are a major source of plasma S1P, we asked whether massive hemolysis of erythrocytes leading to an additional increase in plasma S1P level would trigger mobilization of HSPCs. Furthermore, to shed more mechanistic light on the mobilization of HSPCs in PNH patients and to distinguish the effect of an increase in the S1P chemotactic gradient in PB plasma from the effect of defective retention of HSPCs in the BM microenvironment, we performed mobilization studies in mice exposed to the hemolysis-inducing agent phenhlhydrazine (PHZ) ± blockade of the BM-retaining SDF-1–CXCR4 axis by AMD3100.
Normal C57Bl6 mice were injected with i) PHZ to induce hemolysis and S1P release from erythrocytes, ii) AMD3100 to perturb the SDF-1–CXCR4-mediated retention of HSPCs in BM niches, or iii) both PHZ and AMD3100. Subsequently, we evaluated the number of circulating Sca-1+Kit+Lin– (SKL) HSPCs as well as the number of clonogenic CFU-GM progenitors in PB. In parallel, we evaluated the S1P blood plasma levels by liquid chromatography electrospray ionization tandem mass spectrometry (HPLC ESI MS/MS) and the SDF-1 level by ELISA. In addition, we measured complement cascade (CC) activation by measuring the C5b-C9 (membrane attack complex, MAC) levels.
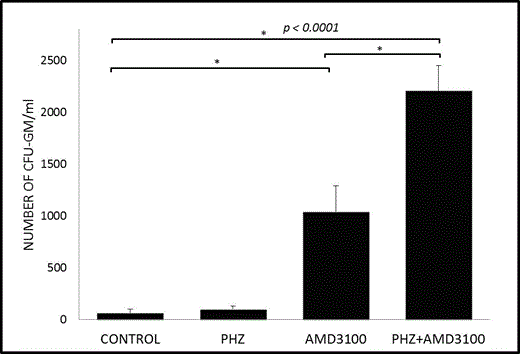
We found that hemolysis doubles the PB plasma level of S1P (from 1 to 2 mM). To assess the effect of plasma S1P versus plasma SDF-1 as chemoattractants mediating egress of HSPCs from BM, we employed plasma derived from control and PHZ-treated mice, W146, a receptor-specific inhibitor for the S1P receptor type 1 (S1P1), and the CXCR4 antagonist AMD3100 in Transwell migration assays. We observed that chemotaxis of BM-purified HSPCs was inhibited by blocking the S1P–S1P1 but not the SDF-1–CXCR4 axes, which demonstrates that the S1P level in plasma is a crucial chemoattractant for HSPCs present under normal steady-state conditions and in PHZ-treated mouse plasma. This observation also clearly shows that the S1P gradient, even under steady-state conditions, is already high enough to promote egress of HSPCs from BM into PB and supports our previous observations that, while the SDF-1–CXCR4 axis plays an important role in retention of HSPCs in BM niches, the SDF-1 plasma level is too low to induce egress of HSPCs (Leukemia 2010;24:976). In our in vivo mobilization studies, we observed that, in contrast to AMD3100 administration, PHZ-induced hemolysis alone had a negligible effect on mobilization of HSPCs, with a peak at 6 h after infusion of this hemolysis-inducing agent (Figure 1). However, when we combined PHZ with AMD3100, mice mobilized twice as many HSPCs as with administration of AMD3100 alone. The degree of mobilization of HSPCs correlated with the free Hb level in plasma and with activation of the complement cascade.
At the steady-state conditions S1P level in PB is already a strong chemotactic factros for BM-residing HSPCs. More importantly, to explain the differential mobilization of PNH-affected HSPCs versus normal HSPCs (Leukemia 2012;26:1722), we show here for the first time that hemolysis alone, even if it doubles the S1P level in PB, requires attenuation of CXCR4–SDF-1-mediated retention in BM niches. Thus, PNH-affected HSPCs, due to defective lipid raft formation, have impaired CXCR4-mediated retention in BM niches and are preferentially mobilized into PB. Finally, our data explain why, compared with PNH, HSPCs are mobilized to a much lesser degree in other hemolytic syndromes (e.g., sickle cell anemia).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

