Abstract
The 2013 version of the European LeukemiaNet (ELN) recommendations for the management of chronic myeloid leukemia (CML) patients defines as optimal response the achievement of at least a partial cytogenetic response (PCyR) and/or BCR-ABL <=10% at 3 months, and of a complete cytogenetic response (CCyR) and/or BCR-ABL<1% at 6 months. Obtaining less than PCyR (i.e. Ph+ 36-95%) and/or BCR-ABL >10% at 3 months, and less than CCyR and/or BCR-ABL 1-10% at 6 months are regarded as warning. Patiens with discordant response between cytogenetic and molecular tests (e.g. PCyR and BCR-ABL >10% at 3 months) may be alternatively considered at the same timepoint as optimal or warning.
To evaluate the outcome of CML patients with discordant results between cytogenetic and molecular tests, we retrospectively analyzed our cohort of early chronic phase CML patients for which both cytogenetic and molecular responses were evaluable at 3 and/or 6 months. All patients received front-line imatinib 400 mg daily. PCyR and CCyR were defined as 1-35% and 0% Ph+ metaphases, respectively; major molecular response (MMR) was defined as BCR-ABL <=0.1%IS. Failure-free survival (FFS) was measured from the start of imatinib to the date of any of the following events: progression to accelerated or blastic phase (ABP), death for any cause at any time, imatinib dose increase (>=600 mg/day) or switch to nilotinib/dasatinib for primary or secondary hematologic or cytogenetic resistance. Cumulative responses and survival probabilities were estimated by the Kaplan-Meier method and compared by log rank test; differences among variables were evaluated by the Fisher's exact test.
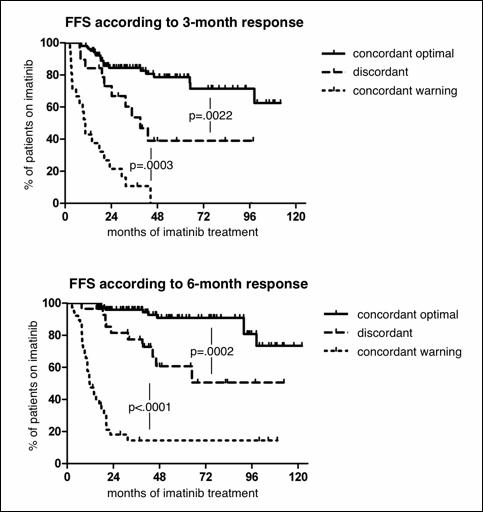
A total of 201 patients were analyzed. Median age at diagnosis was 55 (range 20-84) years. The distribution according to the Sokal score was: 86 (42.8%), 79 (39.3%) and 36 (17.9%) patients for low, intermediate and high risk, respectively. We observed that patients with concordant optimal (n=110) and discordant (n=19) results at the 3 month timepoint had significantly different chances of subsequent 6-month CCyR (88% vs 40%, p<.0001) and 12-month MMR (68% vs 12%, p<.0001), while there were no significant differences between patients with discordant or concordant warning (n=21) results (6-month CCyR 40% vs 14% and 12-month MMR 12% vs 0%, respectively). Also, patients with discordant results, compared to concordant optimal patients, had a significantly longer median time to CCyR (10.5 vs 3.5 months, p<.0001) and to MMR (49.6 vs 9.1 months, p<.0001), while there were no differences between discordant and concordant warning patients. Similarly, considering the 6-month timepoint, patients with discordant (n=28) results had a significantly inferior probability of subsequent 12-month MMR compared to concordant optimal (n=104) patients (16% vs 82%, p<.0001) but not different from concordant warning (n=38) cases (7%). Long-term FFS was significantly different between concordant optimal, discordant and concordant warning patients both considering the 3-month (82.8% vs 52.7% vs 9.6%, respectively) and the 6-month (90.4% vs 67.9% vs 23.7%, respectively) timepoints (figure).
Our results suggest that only CML patients with concordant cytogenetic and molecular optimal response at the earlier timepoints have an excellent probability of obtaining subsequent MMR and a favourable long-term FFS, while patients with at least one warning result should be carefully monitored, since their risk of treatment failure is higher.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.