Abstract
Specific scoring systems developed for patients (pts) with lower-risk myelodysplastic syndromes (LR-MDS) (Garc'a-Manero G et al. Leukemia 2008; Falantes J et al. Clin Lymphoma Myeloma Leuk 2013) are able to identify a significant fraction of pts with a poorer (median OS, 13 months) than expected outcome (Greenberg P et al. Blood 1997). Retrospective data of azacitidine (AZA) in LR-MDS showed hematological improvement and survival when compared to non-responder pts (Lyons R et al. J Clin Oncol 2009; Musto P et al. Cancer 2010). However, the impact of AZA treatment in the group of LR-MDS with poor prognosis by a LR-specific score (LR-S) is uncertain.
To evaluate the impact of AZA treatment in LR-MDS pts with more adverse LR-S by multivariable time-dependent analysis.
Eighty-eight LR-MDS pts (IPSS Low/Int-1 or <10% bone marrow blast with no unfavorable karyotype according to Schanz J et al. J Clin Oncol 2012) with the most adverse specific LR-S were retrospectively analyzed. Patients were separated in two cohorts: Non-AZA cohort (n=61), that included pts who received only best supportive care (BSC; n=46) or BSC plus erythroid stimulating agents (ESA; n=15) at Hospital Universitario Virgen del Roc'o (Seville, Spain) from 2000-2010 who were the core for the development of the LR specific model, versus AZA-cohort (n=27), that included pts treated with AZA (75 mg/m2/day for 5 or 7 days every 4 weeks subcutaneously) in a compassionate use program in Spain
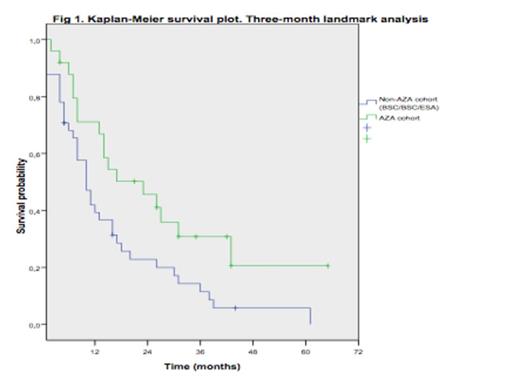
Median age was 71 years (range, 48-86). Patients in the AZA cohort were older and included more RAEB-1, transfusion dependent and elevated LR-S pts. Baseline characteristics and differences between cohorts are shown in Table 1. Median time from diagnosis to AZA therapy was 4 months (range, 0.5-21). At last follow-up, 72 pts (81%) had died (Non-AZA cohort: 55/61; 90% and 17/27; 63% in the AZA group). Median OS for the overall series was 18 months. The actuarial probabilities of OS at 1 and 2 years were 62.4% and 45% for AZA and 25.4% and 11% for Non-AZA cohort (P=10-4). In a multivariable analysis including blast % (<4% vs 4-9%), neutropenia (<0.5 vs >0.5x10e9/L), thrombocytopenia (<50 vs >50x10e9/L) and AZA treatment as time-dependent covariate, the later did not significantly influenced OS (HR, 1.502; 95% CI, 0.258-3; P=0.258) and only severe thrombocytopenia (<50x109/L) showed an independent association with OS (HR, 1.690; 95% CI, 1.036-2.756; P=0.03. Table 2). However, a 3-month landmark analysis showed a survival advantage for pts treated with AZA as compared to non-AZA cohort (median OS, 10m [Non-AZA] vs 23 months [AZA]; P=0.019) and estimated OS rate at 12 and 24 months were 31.5% and 5.7% for Non-AZA vs 50.2% and 41.1% for AZA cohort respectively. Progression to acute myeloid leukemia (AML) occurred in 24.6% (Non-AZA) vs 14.8% (AZA)(P=0.19).
Patient«s characteristics
| Parameter . | Non-AZA (N; %) . | AZA (N; %) . | P . |
|---|---|---|---|
| Age (median) | 70 (48-86) | 74 (62-83) | 0.26 |
| WHO | <0.001 | ||
| RA/RARS | 14 (22.9) | 1 (3.7) | |
| RCMD/RS | 25 (41) | 7 (25.9) | |
| RAEB-1 | 12 (19.7) | 17 (63) | |
| CMML | 10 (16.4) | 0 | |
| LR-MDS score | 0.07 | ||
| 5 pt | 28 (46) | 12 (44) | |
| 6 pt | 27 (44) | 8 (30) | |
| 7 pt | 6 (10) | 7 (26) | |
| Transfusion dependency | 49 (80) | 24 (96) | 0.06 |
| IPSS | 0.01 | ||
| 0 | 3 (4.8) | 0 | |
| 0.5 | 35 (57.1) | 8 (30.4) | |
| 1 | 23 (38.1) | 18 (69.6) | |
| Platelet (<50x10e9/L) | 29 (47.5) | 13 (48) | 0.7 |
| ANC <0.5x10e9/L | 11 (18) | 7 (26) | 0.06 |
| BM blast (4-9%) | 18 (29.5) | 18 (67) | <0.001 |
| Parameter . | Non-AZA (N; %) . | AZA (N; %) . | P . |
|---|---|---|---|
| Age (median) | 70 (48-86) | 74 (62-83) | 0.26 |
| WHO | <0.001 | ||
| RA/RARS | 14 (22.9) | 1 (3.7) | |
| RCMD/RS | 25 (41) | 7 (25.9) | |
| RAEB-1 | 12 (19.7) | 17 (63) | |
| CMML | 10 (16.4) | 0 | |
| LR-MDS score | 0.07 | ||
| 5 pt | 28 (46) | 12 (44) | |
| 6 pt | 27 (44) | 8 (30) | |
| 7 pt | 6 (10) | 7 (26) | |
| Transfusion dependency | 49 (80) | 24 (96) | 0.06 |
| IPSS | 0.01 | ||
| 0 | 3 (4.8) | 0 | |
| 0.5 | 35 (57.1) | 8 (30.4) | |
| 1 | 23 (38.1) | 18 (69.6) | |
| Platelet (<50x10e9/L) | 29 (47.5) | 13 (48) | 0.7 |
| ANC <0.5x10e9/L | 11 (18) | 7 (26) | 0.06 |
| BM blast (4-9%) | 18 (29.5) | 18 (67) | <0.001 |
Multivariate analysis for OS with AZA treatment as time-dependent covariate
| Parameter . | HR . | 95% CI . | P . |
|---|---|---|---|
| AZA treatment | 1.502 | 0.742-3.039 | 0.25 |
| Blasts 4-9% | 1.016 | 0.608-1.698 | 0.95 |
| Thrombocytopenia | 1.690 | 1.036-2.756 | 0.03 |
| Neutropenia | 0.660 | 0.351-1.241 | 0.19 |
| Parameter . | HR . | 95% CI . | P . |
|---|---|---|---|
| AZA treatment | 1.502 | 0.742-3.039 | 0.25 |
| Blasts 4-9% | 1.016 | 0.608-1.698 | 0.95 |
| Thrombocytopenia | 1.690 | 1.036-2.756 | 0.03 |
| Neutropenia | 0.660 | 0.351-1.241 | 0.19 |
<50x10e9/L
<0.5x10e9/L
Azacitidine appeared to increase survival in LR-MDS pts within the most adverse LR-S although differences in OS were not statistically significant in a multivariable time-dependent analysis. Larger number of pts and prospective randomized trials are needed to better address this issue. Thrombocytopenia (<50x10e9/L) is confirmed as the most significant clinical parameter with impact on outcome in LR-MDS.
Off Label Use: 5 azacitidine. Treatment for lower-risk MDS in Europe.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract