Abstract
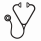
Ruxolitinib is a potent JAK1/JAK2 inhibitor that has demonstrated improved survival, rapid and durable improvements in splenomegaly, disease-related symptoms, and quality of life measures in the 2 phase 3 COMFORT studies in patients with myelofibrosis (MF). Prolonged survival was observed in patients receiving ruxolitinib compared with both placebo (COMFORT-I) and best available therapy (BAT; COMFORT-II). Here, we conducted a pooled analysis of overall survival (OS) in the COMFORT studies.
The COMFORT studies are randomized, phase 3 studies comparing the safety and efficacy of ruxolitinib with placebo or BAT in patients with intermediate-2– or high-risk primary MF, post-polycythemia MF, or post-essential thrombocythemia MF. COMFORT-I is a double-blind study whereas COMFORT-II is open label. Patients initially received ruxolitinib 15 or 20 mg bid based on their platelet counts at baseline (100-200 and > 200 x 109/L, respectively) and were individually titrated to maximize safety and efficacy. The patient populations were generally similar and have been fully described. Patients were allowed to cross over from the control arms of each study upon protocol-defined progression events (primarily progressive splenomegaly, defined as a ≥ 25% increase in spleen volume from baseline or on-study nadir in COMFORT-I and –II, respectively). At the time of analysis, all ongoing control patients had crossed over to ruxolitinib. OS was a secondary endpoint in both studies. Here, OS was analyzed in an intent-to-treat analysis using a multivariate Cox proportional hazards model estimating effects of treatment, study, and IPSS risk (model 1). Effect of baseline spleen volume (per 5 dL) was evaluated using a Cox model adjusting for treatment, study, and IPSS risk (model 2). Two patients in COMFORT without defined IPSS risk status were excluded from model 1; 4 patients without baseline spleen volume measurements were excluded from model 2. For these exploratory analyses, all P values are provided for descriptive purposes only.
Overall, 301 patients were randomized to ruxolitinib (COMFORT-I, n = 155; COMFORT-II, n = 146), and 227 patients were randomized to placebo (n = 154) or BAT (n = 73). In the combined ruxolitinib group, 162 patients (54%) had high-risk MF, and 138 (46%) had intermediate-2 risk at baseline by IPSS criteria compared with 135 (59%) and 91 (40%) patients in the combined control group, respectively.
At this 3-year update, 71 patients (24%) died in the ruxolitinib group compared with 76 patients (33%) in the control group. Survival was consistent across both studies (HR = 1.1; 95% CI, 0.8-1.6; P = .54) and represented an OS benefit in favor of ruxolitinib (Figure A); the risk of death was reduced by 35% with ruxolitinib treatment compared with control (HR = 0.65; 95% CI, 0.46-0.90; P = .01). Patients with intermediate-2–MF had a reduced risk of death compared with high-risk patients (HR = 0.47; 95% CI, 0.33-0.67; P < .0001). Moreover, patients with high-risk MF who received ruxolitinib treatment had a Kaplan Meier–estimated survival similar to that of intermediate-2–risk patients in the control group (Figure B). Among all patients, larger spleen volume at baseline was prognostic for shortened survival; the risk of death increased by 9% for each additional 5 dL in spleen volume at baseline (HR = 1.09; 95% CI, 1.03-1.15; P = .003). Further analysis to evaluate the impact of potentially prognostic and predictive factors at baseline on survival as well as association of other relevant clinical endpoints, such as dynamics of spleen and symptom reduction, with survival will be presented.
Patients who received ruxolitinib in the COMFORT studies had significantly prolonged survival compared with patients who received placebo or BAT. These benefits were seen for both intermediate-2– and high-risk patients, and high-risk patients in the ruxolitinib group appeared to have improved survival similar to that of intermediate-2–risk patients in the control group.
Vannucchi:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees. Hagop:BMS: Research Funding; Novartis: Research Funding; Ariad: Research Funding; Pfizer: Research Funding. Kiladjian:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Sanofi: Honoraria, Membership on an entity’s Board of Directors or advisory committees; AOP Orphan: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Gotlib:Incyte: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Travel support Other. Cervantes:Novartis: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Sanofi Aventis: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau. Mesa:Incyte: Research Funding; Genentech: Research Funding; Lilly: Research Funding; Gilead: Research Funding. Sarlis:Incyte Corporation: Employment, Equity Ownership; Sanofi: Equity Ownership. Peng:Incyte Corporation: Employment. Sandor:Incyte Corporation: Employment, Equity Ownership. Sirulnik:Novartis: Employment. Hmissi:Novartis, A.G.: Employment. Stalbovskaya:Novartis Pharma AG: Employment. Gupta:Novartis: Grant support, Consulting fees and lecture fees Other; Incyte: Grant support, Consulting fees, Grant support, Consulting fees Other. Harrison:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; YM Bioscience: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Celgene: Honoraria; Shire: Speakers Bureau; S Bio: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity’s Board of Directors or advisory committees. Verstovsek:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract