Abstract
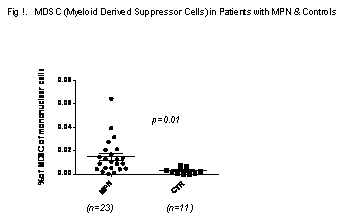
PD-1 and MDSC are two recently discovered important tumor immune escape mechanisms. These immune escape pathways have been studied and their levels were found to be elevated in tumors such as myeloma, pancreatic, renal, ovarian, and breast cancers. Therefore, we studied these two immune escape pathways in patients with myeloid neoplasms including polycythemia vera (PV), Essential thrombocythemia (ET), myelofibrosis (MF) including primary myelofibrosis (PMF) and Post-ET, Post-PV myelofibrosis. We also studied IMID drug in vitro to see if IMID drug mechanism is related to these two escape pathways. 51 patients with MPN and 15 normal volunteer controls were studied. For quantification of expression of PD-1 and PD L-1 in MNC subpopulations (CD4+, CD8+ or CD14+ cells). Ficoll-paque- isolated MNCs were stained with PD-1(CD279-APC) and PD L-1 (CD274-PE)BD Biosciences; CA) plus either CD4-FITC, CD8-FITC or CD14-FITC, along with 7AAD (6 ug/ml), and subjected to flow cytometric analysis. The flow data was analyzed using FlowJo (v 7.6.2). PD-1 or PD L-1 positive cells were expressed as percentage in the subpopulation of cells assayed. For MDSC's, after Ficoll-Paque density centrifugation of peripheral blood, 106 mononuclear cells were stained for flow cytometric analysis using CD11b-APC, CD33-PE, and CD14-FITC (BD Biosceinces; Carlsbad, CA), along with their matched isoptoye controls. 7AAD (6 ug/ml) was used to exclude dead cells to eliminate nonspecific antibody binding. 50,000 events per specimen were acquired and the resultant flow cytometric data was analyzed by FlowJo software. MDSC's were defined as CD11b+CD14-CD33+ cells and were calculated as percentage of viable gated MNC's. MDSC's were also tested for in vitro inhibitory effects on Tcells : MDSC cell sorting was performed by flow cytometry of CD33+ cells from CD11b+CD14- microbead selected cells. CD3 cells were then labeled with CFSE (Life Technologies; CA) and incubated with and without the sorted MDSC's in the presence of CD3+CD28+ microbeads for five days before assessing CFSE activity. For IMID drug in vitro experiments, Pomalidomide (Pm)( Celgene) (10ϰg/ml) was added to mononuclear cells in the presence of CD3+CD28+ microbeads in culture medium. After 5-7 days, cells were assayed for PD-1, PD-L1 expression and MDSC's. Since our data showed no significant difference between ET, PV and MF, we grouped ET, PV and MF together as MPN. The results showed that there is no significant difference between patients with MPN and controls of PD-1 and PD-L1 expression in CD4+, CD8+ and CD14+ cells. However, MDSC's were significantly elevated in patients with MPN compared to controls (Fig 1.). Pomalidomide significantly reduced the expression of PD-1 and MDSC's- expressed as fold change of Pm/DMSO (mean+ SE) in CD4 +,( 0.46 ± 0.120, p= 0.05) , CD8+ (0.66±0.07,p=0.02), MDSC ( 0.71 ± 0.09, P=0.01). Pomalidomide has no significant effect on PD-1 expression in CD14+ cells and PD1-L-1 expression in CD4+, CD8+ and CD14 + cells .Sorted MDSC's significantly suppressed proliferation of the CFSE labeled CD3+ T cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.