Abstract
Innate lymphoid cells (ILCs) are a newly described heterogeneous population of immune cells that can be defined by their expression of specific transcription factors (Tbet, GATA3 or RORgt) and their production of cytokines (IFNg, IL-13, or IL-22). Group 3 ILCs (which can be identified by expression of RORgt and production of IL-22) have been implicated in the maintenance and function of tissues as diverse as liver, gut, lung, spleen and lymph nodes. We have recently described a central role for intrathymic group 3 ILCs (tILC3) in a complex network of endogenous thymic regeneration (Dudakov et al. 2012 Science 336:91-95); a crucial function that allows for renewal of immune competence following infection or immune depletion caused by cytoreductive chemotherapy or radiation injury. In this model, 1) loss of thymic cellularity (and in particular the depletion of CD4+CD8+ double positive, DP, thymocytes) triggers, 2) upregulation of IL-23 by dendritic cells (DCs) which induces, 3) the production of IL-22 by tILC3. Given that IL-22 promotes the survival and proliferation of thymic epithelial cells (TECs), this cascade of events leads to regeneration of the supporting epithelial microenvironment and, ultimately, to rejuvenation of thymopoiesis.
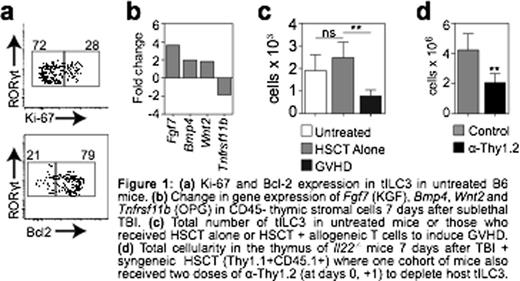
In our previous studies we had demonstrated that, unlike other lymphoid cells, tILC3 were extremely radio-resistant with little if any depletion of cells after even lethal doses of total body irradiation (TBI). Consistent with these findings, here we show that a considerable proportion of tILC3 were non-cycling in steady-state conditions and expressed high endogenous levels of the anti-apoptotic protein Bcl-2 (Fig. 1a). Perhaps unsurprising given their resistance to proliferation-targeted damage, a residual population of host-derived tILC3s could be identified for up to 12 months after syngeneic hematopoietic stem cell transplantation (HSCT). Although at this stage it is unclear if this is because they are very long-lived or if they have the capacity for self-renewal, residual host tILC3 were almost exclusively non-proliferating and expressed high levels of Bcl-2, indicating a quiescent state.
Transcriptome analysis of IL-22 target cells revealed two mechanisms by which IL-22 mediates its effects on TECs; 1) by directly promoting the upregulation of proliferation-associated molecules such as E2f2; and 2) by reducing expression of negative signalling regulators such as Socs3 (an inhibitor of cytokine signalling) and Tnfrsf11b (Osteoprotegerin, a RANKL decoy receptor). This suggests a possible secondary role for IL-22 in promoting enhanced responsiveness to other regenerative factors, such as KGF, BMP4 and RANKL, all of which are increased in the thymus as part of the regenerative response after TBI (Fig. 1b). In our previous studies we found that increased production of IL-22 by tILC3 in response to immune injury was strikingly consistent across several mouse models with lesions in T cell development, including TBI, exposure to corticosteroids, and in mice with genetic mutations. However, one model where this increase in IL-22 does not occur is in the setting of graft versus host disease (GVHD), where tILC3s are profoundly depleted in the thymus (Fig. 1c), likely contributing towards reduced rejuvenation of thymic cellularity and failure to recover during GVHD. Intriguingly, although IL-22 appears to play a considerable role in the regenerative capacity of tILC3, preliminary studies suggest that depletion of tILC3 in IL-22 deficient mice leads to significantly worse recovery compared to Il22-/- mice replete with tILC3 (Fig. 1d). Consistent with this hypothesis of an alternate role in regeneration beyond IL-22 production, production of RANKL is also increased by tILC3 after thymic damage.
Thus, we have identified that tILC3 are highly radio-resistant and long-lived owing largely to their quiescent nature and resistance to apoptosis. These pre-clinical studies focusing on tILC3 biology not only help to identify the mechanisms that allow this nascent cell population to mediate its regenerative effects, but also offer a tantalising glimpse into an alternate pathway mediating their regeneration in the thymus. Taken together, these studies could have the potential to result in novel clinical approaches to enhance T cell immunity in individuals with T cell deficiencies due to aging, infectious disease, chemotherapy or radiation injury.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.