Abstract
Peripheral T-cell lymphomas (PTCL) are a heterogeneous group of non-Hodgkin’s lymphomas (NHL) characterized by poor treatment outcomes with conventional chemotherapy, including CHOP [complete remission (CR) ∼40%]. Retrospective studies suggest that the incorporation of etoposide into chemotherapy regimens may improve outcomes compared to CHOP. In contrast, anthracyclines appear to have a limited role in PTCL, largely attributed to p-glycoprotein related resistance. Pralatrexate was the first drug approved for patients (pts) with relapsed PTCL, providing a rationale to evaluate it in the front-line setting. Herein, we evaluated the efficacy of a novel combination for the primary therapy of pts with PTCL whereby the anthracycline in CHOP backbone was replaced with etoposide (CEOP) alternating with pralatrexate (P).
Pts > 18 years (yrs) with PTCL stages II-IV with no prior therapy and adequate end organ function were eligible. Eligible histologies included, PTCL-NOS, AILT, ALCL (ALK positive pts only allowed if IPI >3). CEOP (Cycle A) was administered as: cyclophosphamide 750 mg/m2 IV d1, etoposide 100 mg/m2 IV d1-3 (or 100 mg/m2 IV d1 and 200 mg/m2 PO days 2-3), vincristine 2 mg IV day 1 and prednisone 100 mg/day X 5 alternating with P (cycle B) 30 mg/m2 IV d 15, 22 and 29 (with vitamin B12 and folate supplementation). Growth factors were used to support both cycles of therapy. Imaging to assess response was done after cycle 2B, 4B and 6B. Pts achieving a remission were eligible for consolidative autologous stem cell transplant (ASCT) after cycle 4B at physician discretion. The primary statistical aim was to improve the CR rate from 40 to 63% with CEOP-P and optional transplant. Secondary objectives included assessment of event free survival (EFS), overall survival (OS) and toxicity of the regimen. A two-stage Simon design (alpha=0.10, 90% power) tested the null hypothesis that the CR rate would be </= to 40%. For the first stage of 20 evaluable pts, the trial would be terminated if 8 or fewer pts experienced a CR after course 2B of chemotherapy. For the second stage, a total of 34 pts were required with at least 17 achieving a CR at the end of therapy to consider the regimen useful.
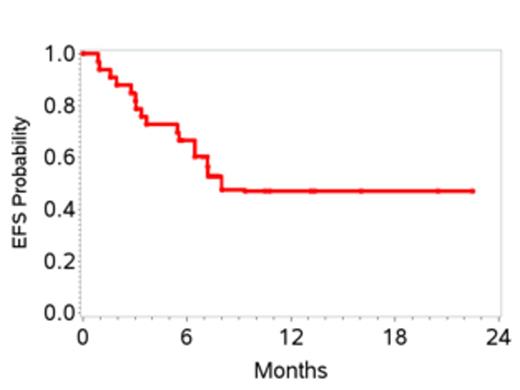
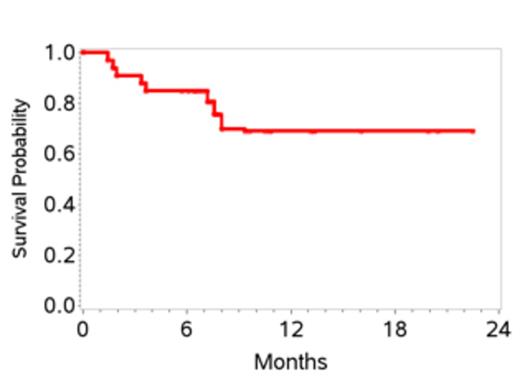
34 pts were enrolled and one withdrew consent before starting therapy, leaving 33 pts enrolled between 7/2011 and 1/2013. Characteristics are shown in Table 1. 27 pts received at least 2 cycles of therapy. 6 pts received only 1 cycle due to early disease progression in 4 and adverse events in 2. Grade 3-4 toxicities attributed to therapy included, anemia (27%), thrombocytopenia (12%), febrile neutropenia (18%), mucositis (18%), sepsis (15%), increased creatinine (12%) and liver transaminases (12%). At the end of stage 1, 10 of 20 pts (50%) achieved a CR; therefore accrual proceeded to stage 2. At the time of this initial analysis, the overall response rate is 70%. To date 15 pts (45%) have achieved a CR with two additional pts pending evaluation. Overall, the 1-yr EFS is 48% (95% CI 28-64), and 1-yr OS 70% (95% CI 47-84) (Figures 1 and 2). 6 pts (18%) to date (n=3 CR, n=2 PR and 1 with stable disease) have received consolidation with ASCT and all are in CR post-transplant. Pts who proceeded to ASCT were younger than the non-transplant cohort (median age 58 versus 64 yrs) with other clinical characteristics being similar. On exploratory bivariate analyses, age <60 yrs, absence of B symptoms, low IPI score (0,1), achieving a CR, and receiving a ASCT were associated with better EFS. Attaining a CR was the only factor associated with better OS and was highest in patients with low IPI (78%) and those with ALCL histology (75%).
Patient Characteristics (n=33)
| . | n (%) . |
|---|---|
| Median age, years (range) | 62 (27 – 83) |
| Karnofsky performance score > 80% | 28 (85) |
| Histology | |
| PTCL- NOS | 21 (64) |
| AILT | 8 (24) |
| ALCL | 4 (12) |
| Stage | |
| II | 4 (12) |
| III | 9 (27) |
| IV | 20 (61) |
| B symptoms | 15 (45) |
| IPI Score | |
| Low | 9 (27) |
| Low – intermediate | 9 (27) |
| High – intermediate | 9 (27) |
| High | 6 (19) |
| Elevated LDH | 16 (48) |
| >2 Extra nodal sites | 9 (27) |
| Median follow-up, months (range) | 8 (6 – 22) |
| . | n (%) . |
|---|---|
| Median age, years (range) | 62 (27 – 83) |
| Karnofsky performance score > 80% | 28 (85) |
| Histology | |
| PTCL- NOS | 21 (64) |
| AILT | 8 (24) |
| ALCL | 4 (12) |
| Stage | |
| II | 4 (12) |
| III | 9 (27) |
| IV | 20 (61) |
| B symptoms | 15 (45) |
| IPI Score | |
| Low | 9 (27) |
| Low – intermediate | 9 (27) |
| High – intermediate | 9 (27) |
| High | 6 (19) |
| Elevated LDH | 16 (48) |
| >2 Extra nodal sites | 9 (27) |
| Median follow-up, months (range) | 8 (6 – 22) |
CEOP-P met the pre-defined stage 1 response criteria. Longer follow-up is needed to assess the impact on the final CR rate and secondary objectives of EFS and OS. Defining optimal front line therapy in PTCL continues to be a challenge and an unmet need.
Advani:Seattle Genetics: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Spectrum Pharmaceuticals: Research Funding. Off Label Use: Pralatrexate is currently only approved for relapsed/refractory PTCL. Lechowicz:Allos Therapeutics: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Millennium: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity’s Board of Directors or advisory committees, Research Funding. Carson:Spectrum Pharmaceuticals: Honoraria, Research Funding, Speakers Bureau. Foss:merck: Research Funding; spectrum: Research Funding; eisai: Membership on an entity’s Board of Directors or advisory committees; millenium: Honoraria, Membership on an entity’s Board of Directors or advisory committees; celgene: Honoraria, Research Funding; seattle genetics: Research Funding. Horwitz:Celgene : Consultancy, Research Funding; Spectrum Pharamaceuticals: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Infinity Pharmaceuticals: Consultancy; Kyowa Hakko Kirn Pharma: Consultancy; Genzyme : Research Funding; Janssen: Research Funding; Millennium: Consultancy, Research Funding. Pro:Spectrum Pharmaceuticals: Honoraria. Pinter-Brown:Spectrum Pharmaceuticals: Consultancy, Honoraria. Smith:Micromet: Consultancy; Seattle Genetics: Consultancy; Celgene: Consultancy; Allos: Consultancy; Genentech: Consultancy; Onyx: Consultancy. Shustov:Spectrum Pharmaceuticals: Consultancy, Honoraria. Savage:Spectrum Pharmaceuticals: Research Funding. Vose:Spectrum Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.