Abstract
The combination of immunomodulatory drugs (IMiDs(r)) and alkylating agents have significantly improved outcomes of patients with relapsed or refractory multiple myeloma (RRMM). Bendamustine, an agent sharing properties of alkylators and purine analogous, and lenalidomide, an immunomodulator drug, are highly effective for the treatment of lymphoprolipherative disorders. Some clinical trials have shown good responses to the combination of bendamustine with steroids and novel agents (thalidomide, lenalidomide and bortezomib) for the treatment of RRMM (Knop S 2005, Lentzsch S 2012, Pönisch W 2013), but further research is needed to confirm the best combination, the optimal schedule of administration, and to better define the safety profile.
A multicenter phase I/II trial tested the combination of escalating doses of bendamustine and lenalidomide, and a fixed dose of dexamethasone (BdL) in repeating 4-weeks cycles as treatment for RRMM. The drug dosages were chosen taking into account the haematological toxicity of both, and that patients were heavily pretreated. The phase I trial was conducted using a 3+3 cohort design beginning at a dose level 0 of intravenous bendamustine 40 mg/m²/day, days 1 and 2, oral lenalidomide 10 mg/day, days 1-21 and oral dexamethasone 40 mg/day, days 1, 8, 15, and 22 every 4 weeks. Patients were treated for 4 cycles + 2 cycles if the response was stable disease (SD) or better. In the phase II study patients were treated at the maximum tolerated dose (MTD) to evaluate the antimyeloma activity of BdL. Endpoints of the study included safety profile, overall response rate (ORR), progression-free survival (PFS), duration of remission (DR) and overall survival (OS).
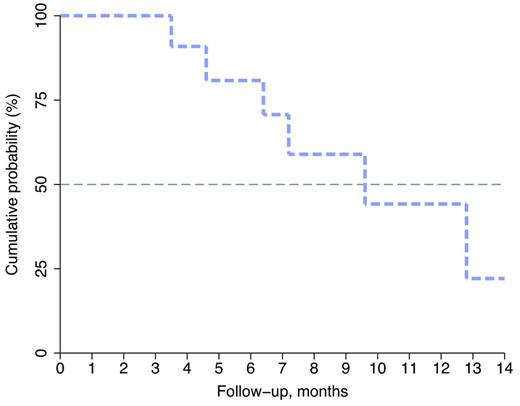
A total of 41 patients (17 in phase I, and 24 in phase II) were enrolled between October 2011 and July 2013. Four patients withdrew their informed consent: 3 before starting treatment and 1 after the fifth cycle. Twenty-eight out of 37 are currently evaluable. Median age of these patients was 68 (43-87 years), with a male/female ratio of 12/16. Sixteen patients had IgG MM, 10 had IgA, and 2 patients had light chain MM. Median number of previous treatments was 3; 9 patients were previously treated with thalidomide and 6 with lenalidomide; 19 with bortezomib and 11 underwent autologous stem cell transplant. The MTD established by the phase I study was dose level 0. To date, with a median follow up of 10 months, ORR is 50% (3 CR, 2 VGPR, 9 PR) and 1 SD. The median OS has not been reached yet. One-year OS is 78% (95% confidence interval [CI], 57%-89%). Median PFS is 10 months (95% CI, 5-12 months) with one-year PFS of 40% (95% CI, 21%-59%). Median duration of remission is 9.6 months with one-year DR of 44% (95% CI, 12%-73%; Figure 1). Toxicity data was available for 32 pts. Grade 3/4 adverse events (AEs) observed in ≥ 10% of patients included neutropenia, thrombocytopenia and anemia. The major non-hematological toxicity was renal dysfunction (6%). A total of 8 pts died during the study: 5 due to toxicity and 3 for progression of disease.
Duration of remission. Kaplan-Meier estimates (in months) with 95% CI of DR in 28 patients treated with BdL for relapsed MM.
Duration of remission. Kaplan-Meier estimates (in months) with 95% CI of DR in 28 patients treated with BdL for relapsed MM.
This study confirms that the combination of bendamustine with lenalidomide and low dose dexamethasone is effective in RRMM patients, even when administered after regimens containing novel agents. The dose of bendamustine in our study is lower (40 mg vs. 75 mg) that of previously published data (Knop S 2005, Lentzsch S 2012, Pönisch W 2013). However, we would like to emphasize that in our both phase I and phase II study we observed a consistent toxicity even utilizing 40 mg of bendamustine and 10 mg of lenalidomide. The toxicity was mainly hematological. Thus far, age, number of previous treatments, or the previous use of alkylating agents did not correlate with observed toxicity. In conclusion with a 50% ORR and 3 CR this is a very promising therapeutic option in RRMM requiring careful management of the patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.