Abstract
Burkitt lymphoma (BL) is the most common form of non-Hodgkin lymphoma that occurs in children and adolescents (Miles/Cairo, BJH, 2012). The 5-year event-free survival (EFS) of children and adolescents with newly diagnosed BL has been significantly improved but for patients with relapsed/refractory disease, the prognosis is dismal due to chemotherapy resistance (Cairo, et al, J Clin Oncol, 2012; Cairo, et al Blood 2007). Novel, non-chemotherapy-based therapies are desperately needed for this specific poor risk population. We have previously demonstrated that CD20 is expressing in ≥98% of pediatric BL (Perkins/Cairo, Clin Adv Hematol Oncol. 2003) and also reported that expanded Peripheral Blood Natural Killer (exPBNK) Cells electroporated with anti-CD20 Chimeric Antigen Receptor (CAR) mRNA have significant cytotoxicity against CD20+ BL in vitro (Chu/Cairo, et al, ASH, 2012).
To examine the anti-tumor effect of anti-CD20 chimeric antigen receptor (CAR+) modified expanded PBNK cells against disseminated CD20+ Burkitt Lymphoma in vivo using human CD20+ BL xenografted NSG mice.
PBMC were expanded with lethally irradiated K562-mbIL15-41BBL cells for 14 days as we previously reported. CD56+CD3- expanded PBNK (exPBNK) cells were isolated using Miltenyi NK cell isolation kit. Anti-CD20-4-1BB-CD3ζ mRNA (CAR mRNA) was produced using the mMESSAGE mMACHINE T7 Ultra kit. CAR mRNA was nucleofected and CAR expression was detected using anti-mouse IgG, F(ab’)2 fragment-specific antibody (Chu & Cairo, et al, ASH, 2012).
5x105 Raji cells expressing luciferase (Raji-Luc) were intraperitoneally (i.p.) injected into the NSG mice (6 weeks, Jackson Labs). Raji engraftment and progression was evaluated using the Xenogen IVIS-200 system (Caliper Life Sciences) after i.p. injection of 150mg D-luciferin/kg/mouse. Photons emitted from luciferase-expression cells were quantified using the Living Image software. After Raji-Luc engraftment was verified in mice at day 7, 5x106 anti-CD20 CAR exPBNK or MOCK exPBNK (no anti-CD20 CAR mRNA electroporation) were i.p. injected to each mouse once a week (day 9,16, 23). Control mice received culture medium instead of NK cells. The cumulative luciferase signals were measured weekly to indicate the tumor growth, dissemination and progression.
Statistical probability of survival and comparison of survival curves were determined by Kaplan Meier Method and the log-rank test. The results with a P value < 0.05 were deemed statistically significant.
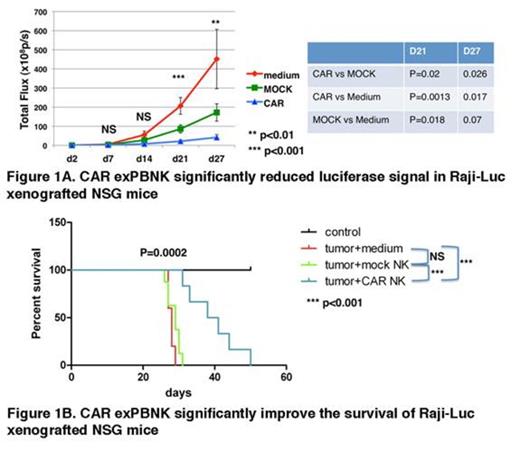
We found that luciferase signals from Raji-luc were not significantly different between the untreated, mock exPBNK treated, and CAR exPBNK treated groups (p=0.0538) after the first NK injection. However, the luciferase signals measured in the CAR exPBNK treated group were significantly different than that in the control mice (two tailed P= 0.0013) and the mock exPBNK treated mice (two tailed P= 0.0229) after the second NK injection. After the third NK injection, the luciferase signals measured in the CAR exPBNK treated group were also significantly different than that in the control mice (two tailed P= 0.0173) and the mock exPBNK treated mice (two tailed P= 0.0256) (Fig. 1A). But luciferase signals were not significantly different between the untreated mice and the mock exPBNK treated mice (two tailed P= 0.0729).
Consistent with the reduced luciferase signals, the CAR exPBNK treated mice had significantly extended survival time with median 40 days compared to the untreated mice (29 days, P<0.001) and the mock exPBNK treated mice (30 days, P<0.001) (Fig. 1B). There was no significant difference of survival between the untreated mice and the mock exPBNK treated mice.
Multiple injections of anti-CD20 CAR mRNA electroporated exPBNK cells can significantly mediate disseminated Raji tumor regression in Raji xenografted NSG mice compared to untreated and mock exPBNK treated mice. Furthermore, anti-CD20 CAR exPBNK significantly increased the survival of Raji xenografted mice. These results indicate therapeutic potential of multiple injections of anti-CD20 CAR mRNA modified exPBNK cells against BL in patients with chemo-immunotherapy resistance. Future directions include examining the anti-tumor activity of CAR+ exPBNK against rituximab/chemotherapy resistant BL in xenografted NSG mice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.