Abstract
Antithymocyte globulin (ATG, Thymoglobulin, Genzyme) derived from New Zealand rabbit immunized with fresh thymocytes has diverse biologic activities against immune response antigens, adhesion and cell-trafficking molecules. These properties are conceptually critical for effective immunosuppression to prevent organ rejection or GVHD. In this study we tested the efficacy of the combination of ATG, tacrolimus (T) and mycophenolate mofetil (M) in the prevention of GVHD in the recipients of allogeneic stem cell transplant from unrelated donor (UHCT).
From June 2008 to December 2012, we performed UHCT in 197 consecutive patients with hematologic malignancies: ALL (N=24), AML (N=85), CLL (N=16), CML (N=8), Hodgkin lymphoma (N=2), MDS/MPN (N=35), non-Hodgkin lymphoma (N=25), myeloma (N=1) and prolymphocytic leukemia (N=1). Patients received either TM (N=121) or ATG-TM (N=76) for GVHD prophylaxis. The distribution of diagnosis between TM and ATG-TM was not different (P=0.72). TM was started on day -3. T dose was 0.03 mg/kg continuous IV daily, M was administered IV at the dose of 10 mg per kg every 8 hours and converted to oral route when patients tolerated oral intake. M was discontinued on day 30 if patients did not develop acute GVHD. ATG total dose of 4.5 mg/kg IV was given daily over 3 days. Stem cell graft was infused within 24 hours of the last dose of ATG. The intensity of the regimen was categorized according to CIBMTR criteria. Disease-risk category was classified as low-risk if CR1, intermediate-risk if CR2 and high-risk if presence of active disease.
ATG-TM cohorts were older, median age 56 vs 52 (P=0.04). Patients with high-risk disease were more likely to receive RIC (P=0.004). There were higher proportion of patients with mismatched graft (P=0.004), and RIC (P=0.005) who received ATG-TM. The remaining other characteristics - HCT-CI score and sex were evenly distributed between TM and ATG-TM. Median follow up for survivors was 20 months.
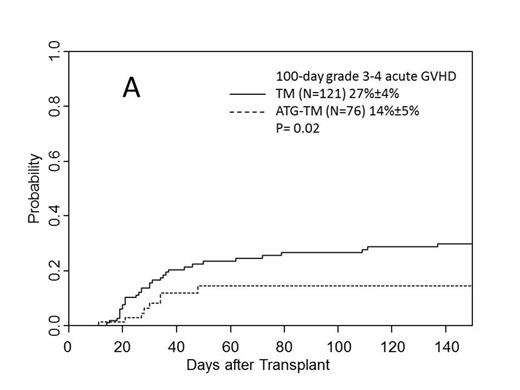
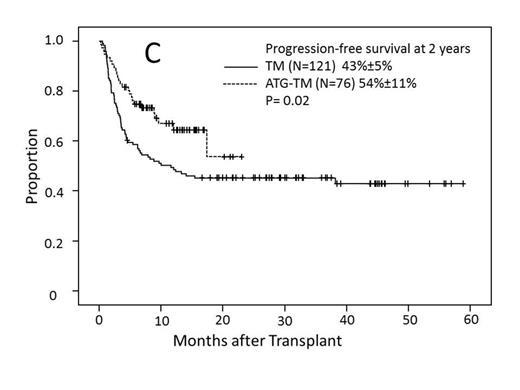
Cumulative incidence of grade II-IV acute GVHD for the TM and ATG-TM cohorts were 49% (95% CI, 39%-59%) and 61% (95%CI, 49%-73%)(P=0.11). Incidence of grade III-IV acute GVHD for the TM and ATG-TM cohorts were 27% (95% CI, 19%-35%) and 14% (95%CI, 4%-24%)(P=0.02) (Fig A). There was no difference in the incidence of relapse or disease progression between TM and ATG-TM cohorts, 16% (95%CI, 8%-24%) vs 23% (95%CI, 0%-47%) (P=0.64). Non-relapse mortality was significantly lower in ATG-TM cohort, 20% (95%CI, 10%-30%) vs 37% (95%CI, 29%-45%) (P=0.01) (Fig B). Cumulative incidence of chronic GVHD was 43% (95%CI, 40% - 72%) in patients who received ATG-TM and 56% (95%CI, 46% - 66%) in TM cohort (P<0.001). Patients receiving ATG-TM had a significantly lower severity of chronic GVHD than patients receiving TM (P=0.004). In univariate analysis, disease-risk status correlated with PFS. PFS at 4 years for low-risk disease was 72% (95%CI, 60%-84%, N=66), 44% (95%CI, 24%-64%, N=24) for intermediate-risk and 36% (95%CI, 24%-48%, N=107) for high-risk disease (P<0.001). PFS at 2 years was better in ATG-TM cohort compared to TM cohort: 54% (95%CI, 32%-76%, N=76) vs 43% (95%CI, 33%-53%, N=121) (P=0.02) (Fig C). Multivariate analysis showed high-risk disease status was independently predictive of poor PFS (P<0.001) and intermediate-risk disease was marginally significant (P=0.05). ATG-TM cohort showed a trend of improved PFS (P=0.07). Disease-risk status at the time of transplant correlated with overall survival (OS) at 4 years: 72% (95%CI, 60%-84%, N=66)) for low risk, 51% (95%CI, 26%-76%, N=24) for intermediate risk and 34% (95%CI, 20%-48%, N=107) for high-risk group (P<0.001), respectively. OS at 2 years was significantly better in ATG-TM cohort: 64% (95%CI, 48%-80%, N=76) vs 49% (95%CI, 39%-59%) (P=0.01) in TM cohort. There were no significant difference among patients who received 9/10 vs 10/10 matched donor, intensity of preparative regimen, and HCT-CI. Multivariate analysis revealed unfavorable impact of high-risk disease and favorable OS in patients who received ATG-TM for GVHD prophylaxis.
ATG dose of 4.5 mg/kg given in conjunction with TM improved the efficacy of GVHD prophylaxis without increased risk of relapse. Lower non-relapse mortality, lower incidence of severe acute and chronic GVHD resulting in improvement of OS and PFS in the ATG-TM cohort.
Off Label Use: Thymoglobulin is approved only for renal transplant rejection. Al-Kadhimi:Genzyme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.