Abstract
Phenotype/genotype correlation can provide important insight into the pathophysiology of hemoglobin disorders and molecular mechanisms regulating globin gene expression. We present 2 unusual patients with β-globin gene cluster deletions of 44 and 589 kb. In the first case, the findings suggest that competition for LCR interaction among β-like globin gene promoters might not play a critical role in ε-globin gene silencing. The second case suggests that other factors in addition to deletion of the putative 3.5 kb silencer element 5' to the δ-globin gene are required for high HbF expression in adults.
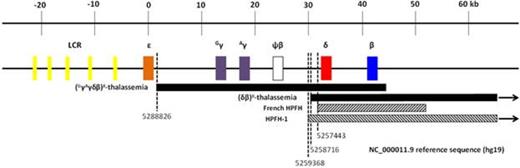
An African American newborn girl presented with marked hepatosplenomegaly, Coombs' negative hemolytic anemia, thrombocytopenia, and direct and indirect hyperbilirubinemia. She was transfused 3 times between birth and at 5 weeks of age. Since then, she maintained a mild anemia with microcytosis without need for further transfusions. At 3 years of age, her Hb was 10.1 g/dL, MCV 57 fL, reticulocyte count 2.0%, serum iron 65, TIBC 309, iron saturation 21%, total bilirubin 0.2. Hemoglobin analysis by HPLC revealed HbA 95.5%, HbA2 3.2%, HbF 1.3%. She did not have mutation in her α- and β-globin gene nucleotide sequences, or any of the common α-globin gene deletions. Multiplex ligation-dependent probe amplification (MLPA) of her β-globin gene cluster revealed that she had a deletion extending from 5' to the Gγ- to 3' to the β-globin genes. The deletion breakpoints were definitively identified by qPCR, followed by gap-PCR and nucleotide sequencing to be from chr11:5,244,999, 1,694 bp 3' to the β-globin gene, to chr11:5,288,826, 755 bp 3' to the ε-globin gene. This novel deletion of 43.8 kb in length removes the Gγ-, Aγ-, ψβ-, δ-, β-globin genes and the 3' β-enhancer, leaving the ε-globin gene and β-LCR intact. A gap-PCR diagnostic test was designed capable of definitive identification of this deletion. This group of (GγAγδβ)0-thalassemia deletions is known to cause neonatal hemolytic anemia of varying severity (Verhovsek et al, Pediatr Blood Cancer 2012;59:941-4) that evolves with age to assume a phenotype of simple β-thalassemia trait but with normal HbA2 level. In addition, mass spectrometry analysis of the hemolysate showed a normal expression profile for the α-globin chains, a decreased expression profile for the β-globin chains and no significant amount of the ε-globin chains. These findings do not support the hypothesis that competition for LCR interaction among β-like globin gene promoters plays an important role in ε-globin gene silencing in adults.
A 65 year old man from Sudan presented with microcytic anemia, Hb 9.6 g/dL, MCV 70 fL, serum ferritin 130. Hemoglobin analysis by HPLC revealed HbA 91.9%, HbA2 2.7%, HbF 5.4%. MLPA revealed a deletion extending from the δ-globin gene to past the β-globin genes. This novel deletion of 588.6 kb in length (from chr11:4,670,164 to chr11:5,258,716) removes the δ- and β-globin genes. The 5' breakpoint of this deletion is 1.3 kb 5' to the 5' breakpoint of the French HPFH, and 652 bp 3' to the 5' breakpoint of HPFH1. Thus this deletion removes almost all of the 3.5 kb intergenic region 5' to the δ-globin gene thought to be important in γ-globin gene silencing in adults (Sankaran et al, N Engl J Med 2011;365:807-14). However, this patient had microcytosis and only moderately increased HbF as in (δβ)0-thalassemia rather than HPFH. These findings suggest that other factors in addition to deletion of the putative silencer, such as the nature of the sequences at the 3' breakpoint of the deletion or other structural features, can influence the occurrence of the HPFH phenotype associated with deletions in the β-globin gene cluster.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.