Abstract
Patients with Wiskott-Aldrich syndrome (WAS) including X-linked thrombocytopenia (XLT) have microthrombocytopenia, and hemorrhage is a major problem. Current management options in WAS/XLT patients include splenectomy, human stem cell transplant (HSCT) and gene therapy. In this study, we asked whether eltrombopag, a thrombopoietin mimetic, would increase platelet counts, improve platelet function, and/or reduce bleeding in WAS/XLT patients.
In 9 WAS/XLT patients and 8 age-matched healthy control subjects, flow cytometry was used to assess platelet function by surface expression of activated GPIIb-IIIa (reported by PAC1) and P-selectin in whole blood after stimulation with low and high concentrations of ADP or thrombin receptor activating peptide (TRAP), and by annexin V binding (a measure of surface phosphatidylserine) in platelet-rich plasma after stimulation with convulxin. Eltrombopag was administered to 5 WAS and 3 XLT patients (50 mg in 2 adults, and 1 mg/kg in 6 children up to 75 mg/day) with a goal platelet count ≥50k.
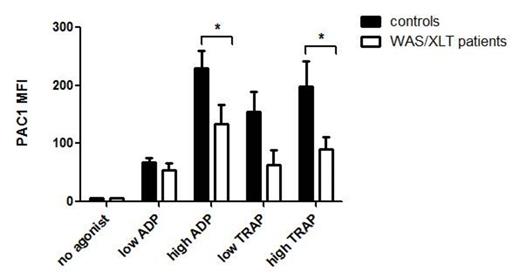
High concentration ADP- or TRAP-induced PAC1 mean fluorescence intensity (MFI) was significantly reduced in WAS/XLT patients compared to healthy controls (Figure). Platelet surface P-selectin MFI in response to TRAP was also significantly reduced. In contrast, annexin V binding to platelets was not different between WAS/XLT and controls. As expected, platelet size of WAS/XLT patients was smaller than controls. WAS protein (which is deficient in WAS/XLT), is important for cytoskeletal movement and could therefore be involved in trafficking of surface proteins. However, surface expression of activated GPIIb-IIIa and P-selectin were no longer different in WAS/XLT patients vs. controls when corrected for size by platelet surface CD41 MFI. In 3 WAS/XLT patients whose platelet count improved on eltrombopag, platelet function did not improve. The table summarizes the results of eltrombopag treatment in 5 responders (2 WAS, 3 XLT patients) and 3 non-responders (3 WAS patients). Comparison of baseline, peak and change in immature platelet fraction in 5 WAS/XLT responders to eltrombopag vs. 7 pediatric chronic immune thrombocytopenia (ITP) patients responding to eltrombopag showed a significant decrease in all three measures, suggesting that platelet production in WAS/XLT patients is more difficult to increase than in ITP patients. Long term eltrombopag use in WAS/XLT patients showed no tachyphylaxis, transaminitis or induction of malignancy.
1) Baseline platelet function in WAS/XLT is reduced compared to healthy age-matched controls, as measured by agonist-induced platelet surface activated GPIIb-IIIa and P-selectin. 2) This reduction is proportional to the reduced platelet size in WAS/XLT compared to controls. 3) In contrast, annexin V binding (a measure of platelet procoagulant activity) showed no differences between WAS/XLT and controls. 4) Eltrombopag has beneficial effects on the thrombocytopenia and bleeding, but not platelet function, in the majority of WAS/XLT patients. 5) This eltrombopag-induced reduction in bleeding is presumably primarily the result of the increased platelet count, but it was also observed in 2 eltrombopag “non-responders” (i.e. patients whose platelet counts did not increase after eltrombopag). 6) The production of new platelets with eltrombopag is less in WAS/XLT than in ITP.
| W=WAS X= XLT . | Age (yrs) . | Clinical History Before Epag . | Reduced Bleeding on Epag . | Baseline Plt Ct (109/L) . | Max Plt Ct on Epag . | 1 = 50% cts ≥ 30k; 2 = 2+ consecutive cts ≥ 20k above baseline; X = neither . | Max Dose (mg) . | Duration of Epag Use (wks) . | Other Therapy On Epag . |
|---|---|---|---|---|---|---|---|---|---|
| Responders | |||||||||
| X | 26 | Severe epistaxis | Y | 19 | 115 | 1, 2 | 75 | 104 | None |
| X | 1.6 | Bruising, petechiae | Y | 20 | 70 | 1 | 39 | 44* | IVIG x1 |
| X | 6 | Epistaxis, ICH; frequent plt tx | Y | 16 | 128 | 1, 2 | 70 | 63* | d/c Amicar |
| W | 2.1 | Frequent plt tx | Y | < 10 | 98 | 1, 2 | 54 | 136* | 2 plt tx; 400 mg/kg IVIG q3 weeks |
| W | 5 | Rituximab, HSCT | Y | ^ | 88 | ^ | 25# | 6* | d/c Prednisone |
| Non-Responders | |||||||||
| W | 12 | Splenectomy Frequent plt tx | N | 5 | 20 | X | 75 | 8 | Frequent plt tx |
| W | 19 | HSCT; ICH x2, retinal + mucosal hemorrhage | Y | 21 | 55 | X | 75 | 36 | 1 plt tx |
| W | 7 | ICH; weekly plt tx | Y | 15 | 22 | X | 75 | 100* | None |
| W=WAS X= XLT . | Age (yrs) . | Clinical History Before Epag . | Reduced Bleeding on Epag . | Baseline Plt Ct (109/L) . | Max Plt Ct on Epag . | 1 = 50% cts ≥ 30k; 2 = 2+ consecutive cts ≥ 20k above baseline; X = neither . | Max Dose (mg) . | Duration of Epag Use (wks) . | Other Therapy On Epag . |
|---|---|---|---|---|---|---|---|---|---|
| Responders | |||||||||
| X | 26 | Severe epistaxis | Y | 19 | 115 | 1, 2 | 75 | 104 | None |
| X | 1.6 | Bruising, petechiae | Y | 20 | 70 | 1 | 39 | 44* | IVIG x1 |
| X | 6 | Epistaxis, ICH; frequent plt tx | Y | 16 | 128 | 1, 2 | 70 | 63* | d/c Amicar |
| W | 2.1 | Frequent plt tx | Y | < 10 | 98 | 1, 2 | 54 | 136* | 2 plt tx; 400 mg/kg IVIG q3 weeks |
| W | 5 | Rituximab, HSCT | Y | ^ | 88 | ^ | 25# | 6* | d/c Prednisone |
| Non-Responders | |||||||||
| W | 12 | Splenectomy Frequent plt tx | N | 5 | 20 | X | 75 | 8 | Frequent plt tx |
| W | 19 | HSCT; ICH x2, retinal + mucosal hemorrhage | Y | 21 | 55 | X | 75 | 36 | 1 plt tx |
| W | 7 | ICH; weekly plt tx | Y | 15 | 22 | X | 75 | 100* | None |
Abbreviations: d/c, discontinue; Epag, eltrombopag; ICH, intracranial hemorrhage; plt tx, platelet transfusion; *, ongoing; ^, missing data; #, increasing dose.
Off Label Use: Eltrombopag was given to WAS/XLT patients for treatment of thrombocytopenia. Michelson:Sysmex: Honoraria. Bussel:GlaxoSmithKline: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Amgen: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Genzyme: Research Funding; IgG of America: Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Eisai: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Symphogen: Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.