Abstract
Three trials, F13CD-1725, F13CD-3760 and F13CD-3720 have investigated the pharmacokinetics (PK) of recombinant FXIII (rFXIII), given at a dose of 35 IU/kg once monthly, in a total of 54 patients with FXIII congenital deficiency (Inbal A et al Blood 2012;119(22):5111-5117; Williams M et al Haemophilia 2013;DOI:10.1111/hae.12224; Kerlin B et al JTH 2013;11(Suppl 2):235-236). The aim of the current analysis was to assess and compare the PK characteristics of rFXIII among trials and 3 different age groups of patients (1-<6, 6-17 and ≥18 years).
All patients were dosed with rFXIII 35 IU/kg every 4th week. Blood samples for PK assessments were collected regularly throughout the dosing interval (28 to 30-day period) in the 3 trials: in the pivotal phase 3 trial, F13CD-1725, at 1 hour, 14 and 28 days post-dose; in the paediatric F13CD-3760 trial at 0.5 and 24 hours and 7, 14, 21 and 30 days post-dose; and in the F13CD-3720 phase 3 extension trial at 1 and 2 hours and 3, 7, 14, 21 and 28 days post-dose. Prior to the PK assessment, all but 2 patients had received treatment with repeated doses of either plasma derived FXIII products or rFXIII, thus all PK measurements were performed at steady-state.
The Berichrom® FXIII activity assay was used for measurement of FXIII activity. PK parameters were calculated using non-compartmental statistical methods, without baseline adjustment. The results include data from a total of 54 patients, aged 1 to 60 years; 41 in the F13CD-1725 trial, 23 in the F13CD-3720 trial (including 16 patients who also participated in F13CD-1725), and 6 in the F13CD-3760 trial.
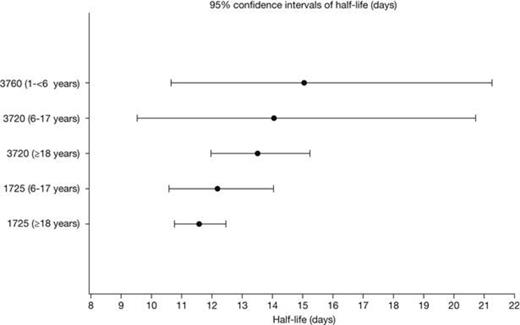
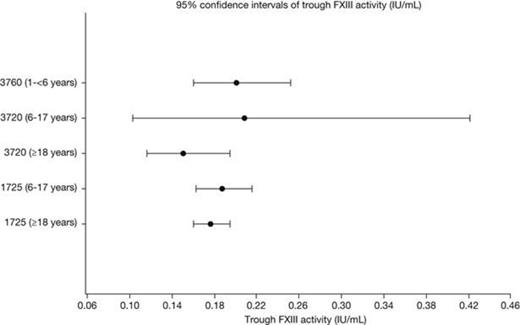
The non-compartmental PK parameters (Cmax, Ctrough, AUC0-28/30d, t1/2) were similar across the 3 age groups (Table 1). Post-hoc, pairwise t-tests across the age groups of log-transformed data did not demonstrate any statistically significant differences, when adjusting p-values for multiple testing. Additionally, separate ANOVA analyses of each of the parameters, showed no significant differences across the groups. The geometric mean half-life ranged from 11.6 to 15.0 days (Figure 1), and the trough FXIII activity levels ranged from 0.15 to 0.21 IU/mL (Figure 2). The geometric mean recoveries were also in the same range; 0.015 (F13CD-1725), 0.013 (F13CD-3760) and 0.020 (F13CD-3720) (IU/mL)/(IU/kg).
Furthermore, it could be demonstrated that the mean PK profiles were similar for the 3 trials, and that Cmax and Ctrough values, as well as FXIII exposures (AUC), were constant over time, based on results from patients participating in both the F13CD-1725 and the F13CD-3720 trial.
PK Parameters for Different Age Groups Across Trials
| Geometric mean (CV)1 | 1-<6 years | 6-17 years | ≥18 years | ||
| Trial ID | F13CD-3760 | F13CD-17253 | F13CD-3720 | F13CD-17253 | F13CD-3720 |
| N | 6 | 15 | 34 | 26 | 204 |
| Body weight, kg2 | 16 (4) | 44 (18) | 55 (32) | 75 (27) | 74 (16) |
| Cmax, IU/mL | 0.67 (20) | 0.71 (38) | 0.81 (27) | 0.76 (21) | 0.88 (22) |
| Ctrough,day 28, IU/mL | 0.205 (22) | 0.19 (26) | 0.21 (29) | 0.18 (22) | 0.15 (42) |
| T1/2, days | 15.0 (34) | 12.2 (25) | 14.1 (16) | 11.6 (18) | 13.5 (27) |
| AUC0-28, IU*h/mL | 2496 (12) | 237 (25) | 258 (22) | 245 (23) | 232 (21) |
| Geometric mean (CV)1 | 1-<6 years | 6-17 years | ≥18 years | ||
| Trial ID | F13CD-3760 | F13CD-17253 | F13CD-3720 | F13CD-17253 | F13CD-3720 |
| N | 6 | 15 | 34 | 26 | 204 |
| Body weight, kg2 | 16 (4) | 44 (18) | 55 (32) | 75 (27) | 74 (16) |
| Cmax, IU/mL | 0.67 (20) | 0.71 (38) | 0.81 (27) | 0.76 (21) | 0.88 (22) |
| Ctrough,day 28, IU/mL | 0.205 (22) | 0.19 (26) | 0.21 (29) | 0.18 (22) | 0.15 (42) |
| T1/2, days | 15.0 (34) | 12.2 (25) | 14.1 (16) | 11.6 (18) | 13.5 (27) |
| AUC0-28, IU*h/mL | 2496 (12) | 237 (25) | 258 (22) | 245 (23) | 232 (21) |
CV= coefficient of variation calculated as (SD/mean)*100
Body weight is presented as mean and standard deviation (SD)
Results based on 3 different time points
In total 16 of the 23 patients in the F13CD-3720 trial were also part of the F13CD-1725 trial, 1 in the age group from 6 to 17 years and 15 in the age group ≥18 years
Based on FXIII activities measured 30 days post-dose
Based on AUC0-30days
The PK profile of rFXIII, after dosing with 35 IU/kg of rFXIII, was independent of age and comparable between trials. It was further demonstrated that FXIII trough activity levels were constant over time, when comparing the individual levels of FXIII activity in patients participating in both the F13CD-1725 and the F13CD-3720 trials. Despite rather large individual variation (CV of up to 38%) in the maximal FXIII activity levels, all individual mean trough activity levels were above 0.1 IU/mL during the entire duration of the trials. The results support that monthly dosing with 35 IU/kg of rFXIII to patients with FXIII subunit A deficiency, regardless of age, is adequate for prophylaxis. Nevertheless individual PK measurements to determine optimal dose and dosing frequency should be considered due to patient variation.
Brand-Staufer:Bayer HealthCare: Travel support, Travel support Other; Pfizer: Membership on an entity’s Board of Directors or advisory committees; Baxter: Membership on an entity’s Board of Directors or advisory committees; Novo Nordisk: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Travel support Other; Bayer: DMC Chair for a Bayer study Other. Carção:Octapharma: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau, Travel Support. Other; CSL Behring: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Travel Support., Travel Support. Other; Pfizer: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Scientific Advisory Board. Travel Support., Speakers Bureau, Travel Support. Other; Novo Nordisk: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau, Travel Support., Travel Support. Other; Biogen: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Travel Support., Travel Support. Other; Bayer : Honoraria, Research Funding, Travel Support. , Travel Support. Other; Baxter: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau, Travel Support. Other. Kerlin:Bayer HealthCare: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Novo Nordisk: Research Funding. Nugent:Novo Nordisk: Honoraria; CSL Behring: Honoraria; Bayer: Honoraria. Will:Novo Nordisk: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity’s Board of Directors or advisory committees. Williams:Novo Nordisk: Membership on an entity’s Board of Directors or advisory committees, Travel support., Travel support. Other; Bayer: Travel support, Travel support Other; Baxter: Membership on an entity’s Board of Directors or advisory committees, Travel support. Other. Rosholm:Novo Nordisk: Employment. Sandberg Lundblad:Novo Nordisk: Employment.
Author notes
Asterisk with author names denotes non-ASH members.