Abstract
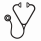
Historically, outcomes after alternative donor (AD)-HCT for FA have been strikingly inferior to those observed in recipients of HLA-matched sibling donor (MSD) hematopoietic stem cells (HSC) with excessive rates of graft failure, graft-versus-host-disease (GVHD), regimen related toxicity and infection, resulting in poor survival rates. Between 2006-2013, 44 FA patients with marrow aplasia and good organ function underwent AD-HCT after total body irradiation 300 cGy (single fraction) with thymic shielding, fludarabine (FLU) 140 mg/m2, cyclophosphamide (CY) 40 mg/kg and antithymocyte globulin (ATG). Outcomes were compared to those transplanted with HSC from an HLA matched sibling donor (n=24) after FLU 175 mg/m2, CY 20 mg/kg and ATG conditioning (1999-2013). GVHD preventative measures were identical with all recipients of marrow having the graft T cell depleted by CD34 selection (regardless of donor type) prior to infusion in addition to cyclosporine A and methylprednisolone or mycophenolate mofetil. Recipients of umbilical cord blood had no additional graft processing.
Except for higher use of pre-HCT G-CSF in recipients of AD-HCT, patient characteristics were similar between the 2 groups. Probabilities of neutrophil recovery, acute and chronic GVHD were similar between the groups (Table 1). Most notably, probability of survival at 3 years was the same between the two groups (Figure 1).
Patient Characteristics and HCT Outcomes
| . | MSD-HCT . | AD-HCT . |
|---|---|---|
| N | 24 | 44 |
| Patient Characteristics | ||
| Age - years, median (range) | 8 (3-43) | 8 (3-34) |
| Male sex - no. (%) | 12 (50%) | 25 (57%) |
| <3 malformations - no. (%) | 13 (54%) | 23 (52%) |
| ≥1 transfusion prior to HCT- no. (%) | 15 (63%) | 18 (41%) |
| G-CSF prior to HCT – no. (%) | 3 (13%) | 21 (50%)* |
| Clonal abnormality prior to HCT – no. (%) | 4 (17%) | 9 (20%) |
| Positive CMV serostatus | 12 (50%) | 27 (61%) |
| HCT Outcomes | ||
| Neutrophil Recovery | 100% | 93% |
| - median time (range) days | 11 (9-41) | 12 (9-40) |
| Grade II-IV acute GVHD | 4% | 9% |
| Grade III-IV acute GVHD | 4% | 0% |
| Chronic GVHD | 8% | 2% |
| Follow-up - years, median (range) | 5.4 (2-11) | 3.3 (1-6) |
| . | MSD-HCT . | AD-HCT . |
|---|---|---|
| N | 24 | 44 |
| Patient Characteristics | ||
| Age - years, median (range) | 8 (3-43) | 8 (3-34) |
| Male sex - no. (%) | 12 (50%) | 25 (57%) |
| <3 malformations - no. (%) | 13 (54%) | 23 (52%) |
| ≥1 transfusion prior to HCT- no. (%) | 15 (63%) | 18 (41%) |
| G-CSF prior to HCT – no. (%) | 3 (13%) | 21 (50%)* |
| Clonal abnormality prior to HCT – no. (%) | 4 (17%) | 9 (20%) |
| Positive CMV serostatus | 12 (50%) | 27 (61%) |
| HCT Outcomes | ||
| Neutrophil Recovery | 100% | 93% |
| - median time (range) days | 11 (9-41) | 12 (9-40) |
| Grade II-IV acute GVHD | 4% | 9% |
| Grade III-IV acute GVHD | 4% | 0% |
| Chronic GVHD | 8% | 2% |
| Follow-up - years, median (range) | 5.4 (2-11) | 3.3 (1-6) |
p value <0.01
To our knowledge, this is the first demonstration of comparable outcomes after AD-HCT and MSD HCT for FA patients with marrow aplasia. These findings have three important implications: 1) timing for HCT need not be delayed if the patient lacks an HLA matched sibling donor, 2) potentially reduced enthusiasm for in vitro fertilization and preimplantation genetic diagnosis to have a 'savior sibling' and 3) ineligibility of FA young patients with a suitable AD (HLA matched adult volunteer or 5-6/6 matched UCB) and good organ function for high risk trials, including gene modified HSC.
Wagner:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract