Abstract
Intensive chemotherapy for adults with newly diagnosed acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) is associated with a considerable risk of morbidity and mortality, commonly due to infections and bleeding. Little information is available on pre-treatment risk factors that predispose to these complications. Here, we investigated the relationship between baseline peripheral blood counts and (a) subsequent adverse events and (b) duration of neutropenia following administration of induction chemotherapy at our institution over the last decade for newly diagnosed AML/MDS undergoing curative-intent induction chemotherapy.
We retrospectively analyzed 205 consecutive adults receiving 7 + 3-like treatment regimens. Daily blood counts were recorded from initiation of chemotherapy until the day of neutrophil recovery (defined as an absolute neutrophil count [ANC] greater than or equal to 500 cells/µL). Adverse events (fever, infection, bacteremia, transfer to the intensive care unit, and death) were recorded until day 35 or administration of additional chemotherapy, whichever came first. Cytogenetic risk was classified based on revised MRC/NCRI criteria. Associations between baseline patient characteristics and adverse events were assessed using Kaplan-Meier survival curves with a log-rank test (for two groups) or log-rank test for trend (for three or more groups); patients requiring salvage therapy for persistent disease were censored on the day such therapy was initiated. Cox proportional hazards models were used to estimate the hazard ratio (HR) for the associations between age, gender, disease type, cytogenetic risk and adverse outcomes in univariate and multivariate analyses.
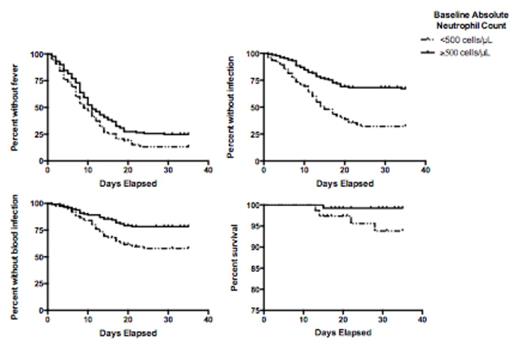
The median age of our study cohort was 53 (range: 18 to 81) years. Sixty-six percent of the patients had de novo AML. Cytogenetic risk was favorable in 14%, intermediate in 60%, and adverse in 24%. Among all patients, the complete remission (CR) rate was 75%, while 2% died before response could be assessed (range: day 13 to 28) and 23% were refractory. When analyzed as a continuous variable, the baseline neutrophil count was statistically significantly associated with fever (HR=0.97 [95% confidence interval: 0.95-0.999] for each increase of 1000 cells/µL, P=0.04) and infection (HR=0.92 [0.87-0.98], P=0.01), but not bacteremia (HR=0.95 [0.89-1.01], P=0.10) or requirement for intensive care unit (ICU) care (HR=1.00 [0.93-1.07], P=0.99). For subsequent analyses, we dichotomized the study cohort based on pre-treatment ANC and compared patients with baseline ANC<500/µL (n=75) with those with ANC greater than or equal to 500/µL (n=130). As shown in Figure 1, baseline ANC<500/µL was associated with the development of fever (P=0.022), documented infection (P<0.001), bacteremia (P<0.001), and death (P=0.035) [Figure 1] but not requirement for ICU care (P=0.39). In patients who achieved CR with 1 course of therapy without myeloid growth factor support, baseline ANC<500/µL was associated with delayed neutrophil count recovery (P<0.0001). In addition to low baseline ANC, we also found that low baseline monocyte and lymphocyte counts were associated with increased risk of documented infection or bacteremia. After adjustment for age, gender, disease type, and cytogenetic risk, the risk of documented infection or bacteremia was 2.71 (1.78-4.12)-fold and 2.11 (1.26-3.55)-fold higher in patients with baseline ANC<500/µL. Having demonstrated that baseline ANC<500/µL was associated with both duration of neutropenia and the development of adverse events and death, we wondered whether this patient subset could be suitable for the targeted use of myeloid growth factors. To investigate this, we evaluated whether those with 0% bone marrow blasts at first post-treatment assessment were also more susceptible to adverse events after day 14. Indeed, this was the case for infection (P=0.0140) and bacteremia (P=0.0023).
Our studies identify pre-treatment ANC<500/µL as a risk factor for both infection-associated adverse events, including death, and longer duration of neutropenia. If validated, these results would provide the rationale for the risk-adapted testing of myeloid growth factor support in patients with AML/MDS with pre-treatment ANC< 500/µL when undergoing curative-intent induction chemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.