Abstract
InO is a humanized anti-CD22 antibody conjugated to calicheamicin. CD22 is expressed on a majority of B-cell ALL. An initial study suggested InO efficacy and tolerability in ALL (Cancer. 2013 Aug 1;119(15):2728-36).
To optimize the InO dose and weekly schedule, and evaluate safety and efficacy in CD22+ relapsed/refractory ALL.
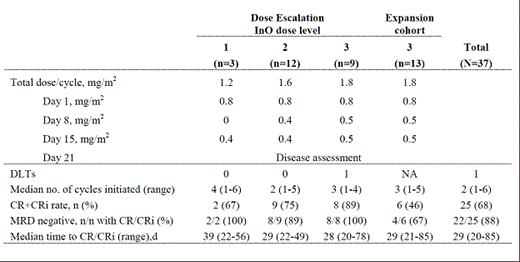
This phase 1, dose-escalation and expansion study enrolled pts aged ≥18 y with CD22+ refractory/relapsed ALL and no central nervous system disease. InO was administered in 28-d cycles (Table), up to 6 cycles. The final dose was to be determined based on dose-limiting toxicities (DLT) and efficacy, using the MDACC EffTox V2.10 software. Adverse event (AE) severity was assessed per CTCAE V3. DLTs included the following InO -related events in Cycle 1: gr ≥4 nonhematologic AE; prolonged myelosuppression; gr 3 nonhematologic AE >7 d; any AE resulting in permanent InO discontinuation. Complete response (CR) was defined as <5% bone marrow blasts without peripheral blasts, ANC ≥1,000/µL, platelets >100,000/µL and no extramedullary disease; CRi defined as CR without ANC or platelet recovery.
We report data for 37 pts: median age was 56 y (23-75 y); 65% were male; 17 (46%) pts were in salvage 1, 9 (24%) in salvage 2, and 10 (27%) in salvage ≥3; 7 (19%) pts had prior allogeneic stem cell transplant (SCT); 6 (16%) pts were Ph+; CD22 found on a median of 98% blasts (31.1-100%); median WBC was 5.18×103/mm3 (0.5-67.2×103/mm3). The expansion cohort (n=13) was comprised of pts with higher peripheral blast counts (PBC) and higher risk cytogenetics as compared to the dose escalation group (n=24): median PBC of 4158/µL (0-38,976/µL) and 18/µL (0-10,189/µL), respectively; aberrant baseline cytogenetics were reported in 10/13 (77%) expansion pts including 2 pts Ph+ and 2 pts t(4;11) and 11/24 (46%) escalation pts. Median follow-up in surviving pts was 4.1 mo (1-12.6 mo). Thirty-two pts discontinued InO: 14 due to PD, relapse or resistant disease, 11 proceeded to SCT, 6 due to AEs (1 pt each: gr 3 acute renal failure, gr 2 ascites, gr 2 increased gamma-glutamyl transpeptidase (GGT), gr 5 graft failure, gr 2 constitutional symptoms and gr 2 veno-occlusive disease (VOD)) and 1 to receive maintenance therapy. InO-related ≥gr 3 AEs (≥10% of pts) were thrombocytopenia (30%) and neutropenia (19%). Other ≥gr 3 hepatic AEs included increased ALT (5.4%) and increased GGT (3%). Gr 2 AEs include ascites (2 pts) and VOD (2 pts). Sixteen deaths were reported: ALL (n=11), sepsis following SCT (n=3), graft failure (n=1) and gut GVHD and liver dysfunction (n=1). The RP2D was determined as 1.8 mg/m2/cycle based on efficacy and safety in the dose escalation patients (1 DLT; 89% CR+CRi rate; all remissions were minimal residual disease (MRD) negative (<1 blast out of 104 mononuclear cells by flow cytometry) with a dose reduction to 1.6 mg/m2/cycle upon achievement of CR/CRi. The dose reduction was recommended due to AEs leading to discontinuation and the observation of increased InO exposure with successive cycles in prior studies.
The remission rate (CR+CRi) for the dose escalation pts (n=24) was 79% (95% CI: 58, 93) and 46% (95% CI: 19, 75) for the dose expansion pts (n=13); 18/19 (95%) escalation pts with CR/CRi and 4/6 (67%) expansion pts with CR/CRi achieved MRD negativity. Overall, the median time to remission and MRD negativity was 29 d (20-85d) and 34 d (22-141d), respectively. Minimum InO concentrations in responders were higher than in pts failing treatment.
The RP2D was confirmed as 1.8 mg/m2/cycle, InO had a tolerable safety profile primarily characterized by hematologic, gastrointestinal and hepatic AEs. Single-agent InO demonstrated encouraging clinical activity in this relapsed/refractory population; preliminary efficacy results appear dynamically related to exposure and circulating blasts. Further exploration in CD22+ ALL is ongoing.
DeAngelo: Pfizer Inc: Membership on an entity’s Board of Directors or advisory committees. Off Label Use: This abstract presents findings from a phase I study of inotuzumab ozogamicin in patients with relapsed/refractory CD22+ acute lymphoblastic leukemia; this drug is investigational and is not approved for use in any indication in any country. Stock:Pfizer Inc: Research Funding. Liedtke:Millennium: The Takeda Oncology Company: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Schiffer:Amgen Inc: Research Funding; Pfizer Inc: Research Funding. Ananthakrishnan:Pfizer Inc: Employment. Boni:Pfizer Inc: Employment. Luu:Pfizer Inc: Employment. Liau:Pfizer Inc: Employment. Vandendries:Pfizer Inc: Employment. Advani:Pfizer Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract