Abstract
ATRA and anthracycline-based chemotherapy is a standard remission induction therapy for APL, leading to complete remission (CR) rate of 90% or more. (Asou et al, 2007; Ades et al, 2010; Avvisati et al, 2011). As reported in many APL studies including the JALSG study, risk adopted strategy according to initial leukocyte counts has demonstrated successful results. However, the long-term outcome of the patients with initial leukocyte counts < 3,000/µl received ATRA alone in the induction therapy and followed by post remission chemotherapies, remains to be elucidated. Furthermore, it is controversial whether concomitant chemotherapy is needed for such a very low risk group. In the JALSG-APL97 study, patients with initial leukocyte counts < 3,000/µl received ATRA alone until remission (Group AA), except for patients with leukocytosis during the ATRA therapy who received additional chemotherapy (Group AD). Here, we reported the long-term outcome of this study based on risk adopted therapy, particularly, focusing on the outcome of the very low risk group.
The treatment schedule of JALSG-APL97 study was initially reported by Asou et al. in 2007. In brief, patient groups were defined as: leukocytes < 3000/µl (Group A: ATRA alone), 3000/µl ≤ leukocytes < 10,000/µl (Group B: ATRA plus IDA/Ara-C: 2+5), and leukocytes ≥ 10,000/µl (Group C: ATRA plus IDA/Ara-C: 3+5). Patients who experienced leukocytosis received additional chemotherapy (Group D). After 3 courses of consolidation chemotherapy, patients achieved molecular CR were allocated to an intensive chemotherapy group or observation. The CR rate, overall survival (OS), event-free survival (EFS), and cumulative incidence of relapse (CIR) were analyzed for each group.
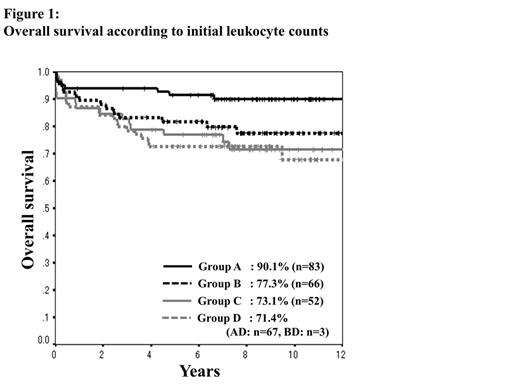
Two hundred and seventy-one newly diagnosed APL patients, ranging from 15 to 70 years of age, were evaluable. The number of patients in each group was 150 (A), 69 (B), 52 (C) and 70 (D), respectively. Of 150 patients in Group A, 83 achieved CR with ATRA alone (AA), and 67 were added chemotherapy due to leukocytosis during ATRA therapy (AD). In Group A, B, C and D, CR rates were 95.2%, 97.0%, 90.4% and 97.1%, respectively (P = 0.30); OS (Figure 1) 90.1%, 77.3%, 73.1% and 71.4%, respectively (P = 0.02); EFS (Figure 2) 71.1%, 63.6%, 55.7% and 64.3%, respectively (P = 0.26); CIR 23.5%, 27.6%, 34.5% and 20.4% (P = 0.51), respectively. Initial leukocyte counts in Group AA were significantly lower compared to those in Group AD (median leukocyte counts; 900/µl vs. 1,100/µl, P = 0.03). The median administration period of ATRA was similar between Group AA and AD (46 days vs. 43 days, P = 0.57). Differentiation syndrome was more frequent in Group AA (28.0% vs. 14.9%, P = 0.04). The CR rate and early death rate were not different between two groups (95.2% vs. 95.5%, P = 0.92 and 3.6% vs. 4.5%, P = 0.79, respectively). OS was significantly inferior (90.1% vs. 73.1%, P = 0.005) and non-relapse mortality after post-remission therapy was significantly higher in Group AD (5% vs. 16%, P = 0.04), compared to Group AA, while EFS was not different between two groups (71.1% vs. 65.7%, P = 0.33). The cumulative incidence of late relapse occurred more than 2 years after CR was significantly higher in Group AA compared to Group AD (17.5% vs. 3.9%, P = 0.04).
Risk adopted therapy according to initial leukocyte counts is totally useful in this study as well as previous reports including us. OS was favorable in APL patients with initial leukocyte counts < 3,000/µl, achieved CR by using ATRA alone for remission induction therapy, whereas EFS in this group was still unsatisfactory in the long-term follow up. It could be explained by the high frequency of late relapse. Ades et al reported better long-term outcomes in patients concomitantly treated with ATRA and chemotherapy rather than in those treated with ATRA followed by chemotherapy in their APL patients with initial leukocyte counts < 5000/µL. However, very low risk patients (Group AA) could be put into the separate category, and therapeutic approaches to reduce the late relapse in this group should be discussed. Additionally, the genetic profile studies will provide us informative data in relation to initial leukocyte counts and leukocytosis during ATRA therapy.
Kiyoi: Bristol-Myers Squibb: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Novartis Pharma: Research Funding; Kyowa Hakko Kirin Co. Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.