Abstract
Recent data indicate that the survival of chronic myeloid leukemia (CML) patients, who are in Major Molecular Response with TKI, is not statistically significantly different from that of the general population and that a subgroup of patients who experienced long-term Complete Molecular Responses can stop therapy without relapse. However, less is known regarding the actual impact of TKI therapies or their cessation on patients’ health-related quality of life (HRQoL).
We conducted a monocentric observational quality of life study on CML patients having received or currently receiving Imatinib, Nilotinb or Dasatinib, in order to investigate the relationships between HRQoL and treatment or treatment interruption.
The analysis was performed on 110 CML patients diagnosed and recruited from 1999 to 2012 in our department and followed for more than 3 months. At the time of HRQoL assessment (median duration from diagnosis: 5.6 years; interquartile range [IQR] 2.4-10.1), 8.8% of patients had stopped TKI therapy for more than 3 months because of long-time CMR, 49.6% were treated with Imatinib, 22.1% with Dasatinib and 19.5% with Nilotinib. The total number of different prescribed TKIs in the course of the disease was 1 in 60.2% of the cases, 2 in 23% and 3 in 16.8%. HRQoL was assessed with the 36-Item Short-Form Health Survey (SF-36), using age-sex adjusted standardized scores expressed as standard deviation [SD] from the French general population reference values for age and gender. Key socio-demographic and clinical data including age, gender, education, Sokal risk, response to therapy and duration of treatment, smoking, obesity, hypertension, diabetes, dyslipidemia, number of medications, ECOG performance status, ADL/IADL and Mini Nutritional Assessment scale (MNA) were also taken into account. Univariate and multivariate linear regression analyses were used to identify independent predictors for each SF-36 subscale and summary scores (Physical [PCS] and Mental Composite Scores [MCS]).
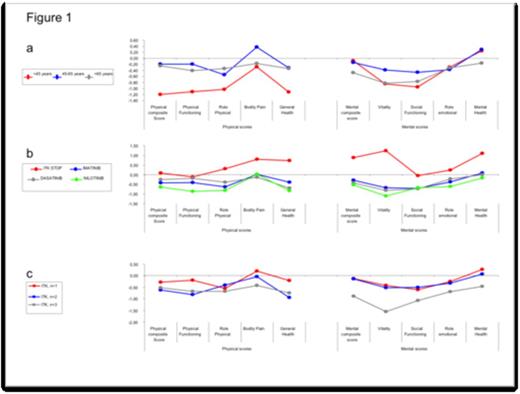
In univariate analysis, factors significantly associated with lower PCS scores included younger age (under 45) (-1.20 SD), lower education level (-0.79SD), obesity (BMI>30) (-1.39SD), pre-existence of dyslipidemia (-1.57SD), ADL with more than 1 limitation (-0.98SD), ECOG >1 (-1.83SD), MNA at risk or poor nutritional status (-1.26SD); factors associated with lower MCS scores were pre-existence of dyslipidemia (-0.92SD), ECOG>1 (-1.31SD), MNA (-0.87SD), current CML treatment (-0.28SD [Imatinib],-0.42SD [Sprycel] and -0.53SD [Nilotinib]) and more than 2 lines of TKI (-0.89SD). In multivariate analysis, only younger age (p=0.009) and dyslipidemia (p=0.023) were negatively correlated to PCS and current CML treatment (p=0.001) and more than 2 TKI (p=0.013) negatively correlated to MCS. In figure 1, we report the standardized SF-36 scores of CML patients according to age (1a), treatment (1b) or treatment lines (1c).
Age-sex adjusted and standardized SF-36 scores of CML patients, expressed as standard deviations (SD) from the French population reference values
Age-sex adjusted and standardized SF-36 scores of CML patients, expressed as standard deviations (SD) from the French population reference values
We confirm previous data indicating worse HRQoL in younger CML patients treated with Imatinib. In our study, this effect was also observed with 2nd-generation TKIs. Our findings were in the same order of magnitude as previously reported (Efficace et al, blood, 2011).
We failed to demonstrate any major differential effect between the different TKI (Imatinib, Nilotinib or Dasatinib) on HRQoL suggesting that the choice of TKI therapy cannot be determined by this criterion. Moreover, comparing the number of TKI changes, we failed to demonstrate any effect of “only-one” change of TKI on HRQoL,. This suggests that one change in CML therapy does not worsen QOL , whereas a drastic decrease in mental HRQoL scores was found in patients receiving more than two lines of TKI.
The most relevant finding was that patients who benefited from TKI interruption because of stable complete molecular remission had better mental HRQoL outcomes, suggesting that TKI interruption could have a positive impact on HRQoL and hence has to become the objective to achieve in CML to normalize HRQoL.
Giraudier:NOvartis: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria. Tulliez:Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.