Abstract
Survival of patients with primary myelofibrosis (PMF) can be defined according to the IPSS/DIPSS model, which stratifies patients by the following risk factors: age over 65 years, hemoglobin lower than 10 g/dL, leukocyte count over 25 x109/L, blast cells equal to or higher than 1%, presence of constitutional symptoms. (Passamonti et al, Blood 2010). No information on the survival of patients with secondary MF is available so far. Ruxolitinib is an oral JAK1/2 inhibitor approved by FDA and EMA for the treatment of patients with MF (PMF and secondary MF). Overall survival was a secondary endpoint in the COMFORT-1 (ruxolitinib vs. placebo) and COMFORT-2 (ruxolitinib vs. best available therapy) phase III studies. Despite the crossover design and the intention to treat analysis model, patients randomized upfront to ruxolitinib had a better survival with an HR of 0.58 in COMFORT-1 and of 0.48 in COMFORT-2 (Verstovsek et al, ASH 2012, abs 800; Vannucchi et al, EHA 2013, abs S1111). In addition to the prospective data from these phase III trials, two comparisons of outcomes from the phase I/II experience with historical controls are also available. One compared survival of MF patients treated with ruxolitinib to that of an historical cohort at the same institution without evidence of advantage (Tefferi et al, NEJM 2011). The second demonstrated survival benefit for patients treated with ruxolitinib when compared to a multicenter matched historical cohort (Verstovsek et al, Blood 2012). In those studies, survival time was assessed from the beginning of ruxolitinib treatment to death/last contact and included patients with PMF and secondary MF.
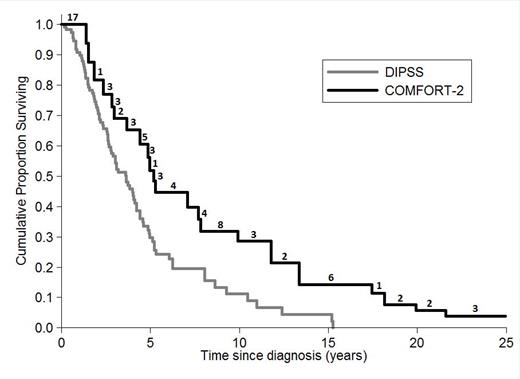
The aim of this study was to compare survival from diagnosis of COMFORT-2 patients with PMF who received ruxolitinib (date of diagnosis was extracted from medical documentation in the study) with survival from diagnosis of patients with PMF collected in the DIPSS database (multicenter study, conventionally treated 519 patients). We included 77 PMF patients from COMFORT-2 (excluding secondary MF) and 111 PMF patients from DIPSS with the same entry criteria of COMFORT-2 at diagnosis (excluding DIPSS low risk and intermediate-1, WBC <1500 x109/L, PLT <100 x109/L, circulating blast cells >10%; spleen<5 cm from LCM). Backdating COMFORT-2 data to the date of diagnosis generates left-truncated data: potentially eligible patients dying before they had the chance to enter the COMFORT-2 study were excluded. As a consequence, standard survival methods may lead to biased results in this retrospective comparison. To avoid bias, Kaplan-Meier estimates and other statistical methods for left-truncated (and right censored) data were applied (Klein and Moeschberger, 2003). One COMFORT-2 patient (alive at the date of last follow-up) was excluded from the analysis because the date of initial diagnosis was not available. In addition, age at initial diagnosis was calculated and included as a covariate in a multivariate Cox regression.
Survival estimate from diagnosis of PMF patients according to COMFORT-2 (n=76) and DIPSS cohort (N=111). Numbers above the COMFORT-2 curve represent the number of patients entering ruxolitinib treatment at each time point during follow-up.
Survival estimate from diagnosis of PMF patients according to COMFORT-2 (n=76) and DIPSS cohort (N=111). Numbers above the COMFORT-2 curve represent the number of patients entering ruxolitinib treatment at each time point during follow-up.
In conclusion, comparing survival from diagnosis in PMF patients led to results consistent with those of the two phase III trials. Patients who introduced ruxolitinib at some point during their disease history (COMFORT-2) had a better survival when compared to those who continued standard treatments for the whole follow-up (DIPSS).
Knoops:Novartis: Consultancy; BMS: Consultancy. Hollaender:Novartis: Employment. Squier:Novartis: Employment. Harrison:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; YM Bioscience: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Celgene: Honoraria; Shire: Speakers Bureau; S Bio: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.