Abstract
The myeloproliferative neoplasms (MPN), in particular myelofibrosis, are associated with elevated levels of inflammatory cytokines and constitutional symptoms. Treatment with JAK inhibitors (JAKi) have lead to marked improvement in symptoms and splenomegaly. Signaling through the JAK pathway is critical for T cell development and differentiation. However the baseline immune signature remains largely undescribed in MPN as does the effect of JAK inhibition on the immune subsets in this disease.
The % and absolute number of CD4+ T cell subsets (TH1, TH2 and TH17 and Foxp3+ T regulatory cells) in peripheral blood (PB) were investigated by flow cytometry. T cells were stimulated and stained intracellularly for IFNg, IL-4, IL-17 & TNFα. Tregs were defined as CD4+ CD25highCD27+FOXp3+. The serum level of 30 cytokines was also measured by Luminex. Patients received either ruxolitinib (n=21) or SAR302503 (n=13) as JAKi.
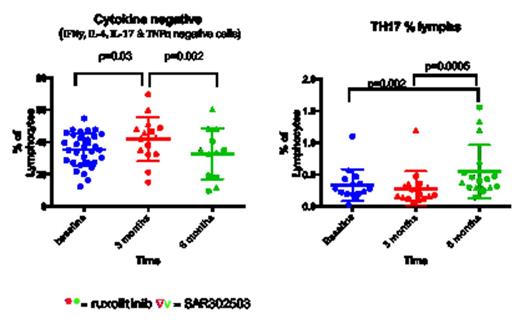
We analysed 50 MPN patients (30 Myelofibrosis, 15 Polycythemia Vera, 5 Essential Thrombocythemia) and 14 healthy donors (HD). 34 patients were treated with JAKi and sequential PB samples were obtained at 1, 3, 6 and 12 month intervals (median follow up 6 months). Tregs are significantly lower in MPN patients compared to HD and drop further following treatment (p<0.0001 and p=0.0049 respectively). There was no difference at baseline in the T effector subsets between the groups including TH1, TH2 and TH17 secreting cells but there was a significant increase in TH17 following JAKi therapy (fig 1a). JAKi resulted in a significant decrease (p=0.03) in CD4 T cells secreting pro-inflammatory cytokines at 3 months follow up although this was less evident at 6 months follow up and occurred irrespective of disease response to treatment. This silencing was confirmed by both intracellular staining and luminex assay of supernatants including a significant decrease in Interleukin-2 receptor (IL-2r) p=0.0007, Interferon gamma induced protein (IP-10) p=0.0006, monokine induced by gamma interferon (MIG) p=0.0008 and hepatocyte growth factor (HGF) p=0.0009.
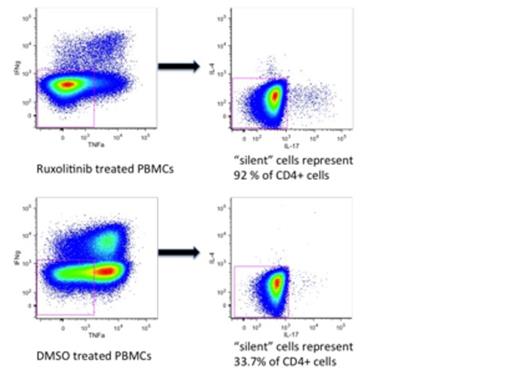
This finding was reproduced in-vitro in healthy peripheral blood mononuclear cells (PBMCs). PBMCs were treated with the JAKi ruxolitinib (100-300NM) in the presence or absence of plate bound anti-CD3/28 stimulation and cultured for 5 days. Tregs were reduced in number and there was a considerable increase in the percentage of “cytokine negative” or “silent” T effector cells by FACS analysis compared to untreated or vehicle treated cells (median of 42 % of CD4 to 91% of CD4) (fig 1b). This finding was reproduced by Luminex cytokine assay of supernatants. Western blot demonstrated a reduction in pSTAT3 in ruxolitinib treated cells. To assess the effect of JAKi on Treg function, healthy isolated Tregs were treated with ruxolitinib and co cultured with CFSE labeled autologous T effector cells. Short term JAKi treated Tregs were unable to suppress the proliferation of T effector cells compared to Tregs treated with vehicle alone. Similarly, proliferation rate and function of Tregs was reduced following 4 weeks expansion in the presence of ruxolitinib compared to expanded Tregs in the presence of ATRA and rapamycin as a control.
Tregs are significantly lower in MPN patients compared to healthy controls in keeping with the inflammatory environment of MPN and decrease further with JAKi. Surprisingly, T effector numbers were not significantly different to healthy controls at baseline and TH1 and TH2 subsets did not change with therapy. However, secretion of proinflammatory cytokines from these cells was blocked with JAKi both invivo and invitro resulting in a functional silencing of T effectors. Interestingly, TH17 subsets increase with treatment possibly representing a polarization from a Treg phenotype to a TH17 phenotype, suggesting the re-establishment of immune-surveillance against the malignant clone. Further investigation is required to confirm the hypothesis that these expanded TH17 cells originate from Tregs or previously “silenced” CD4 T cells.
Harrison:Novartis: Honoraria; Sanofi : Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.