Abstract
Activating KIT mutations are a hallmark of human systemic mast cell disease and have also been identified in gastrointestinal stromal tumors, AML, melanoma, and seminoma. Traditionally, constitutively active KIT mutations have been targeted by tyrosine kinase inhibitors; however, currently available inhibitors have limited activity against resistant mutations and sustainable responses are often not achieved. Our goal has been to identify a synergistic drug combination that increases the effectiveness of KIT inhibitors. A recent study in chronic myelogenous leukemia reported enhanced anti-proliferative/pro-apoptotic effects when combining a BCR-ABL inhibitor with inhibitors targeting a novel Wnt/Ca++/Calcineurin/NFAT pathway. Based on shared downstream signaling mechanisms between BCR-ABL and KIT kinases we hypothesized that a similar combination would work to target KIT mutant kinases.
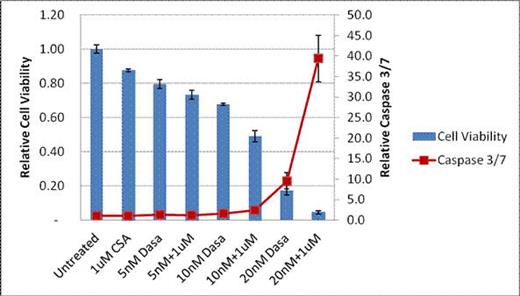
Cell viability and caspase 3/7activity following 48hrs of treatment in murine P815 mastocytoma cell line
Cell viability and caspase 3/7activity following 48hrs of treatment in murine P815 mastocytoma cell line
The activity of NFAT transcription factors is known to be regulated by the phosphatase activity of calcineurin. To confirm our results were calcineurin- and NFAT-dependent, we repeated the previous combination experiments with the NFAT specific inhibitors, rocaglamide and tributylhexadecylphosphonium bromide (THPB). We observed a synergistic decrease (average CI value 0.81 for rocaglamide and 0.43 for THPB) in cell viability and increase in apoptosis when combining these NFAT-specific and KIT kinase inhibitors, indicating NFAT as an important mediator of the observed synergy. Next we characterized NFAT in KIT mutant mast cells and found constitutive NFAT activation in each cell line, as indicated by nuclear localization and dephosphorylation under unstimulated conditions. NFAT-dependent transcriptional activity, as measured by a luciferase reporter system, decreased following treatment with either calcineurin or NFAT inhibitors. Unexpectedly, NFAT-dependent transcriptional activity also decreased following treatment with a number of KIT inhibitors. However, only calcineurin inhibitors and not KIT inhibitors, completely blocked NFAT activation following calcium influx, suggesting a unique mechanism by which KIT inhibition affects NFAT-dependent transcriptional activity. To determine downstream effectors of the observed synergy, we measured changes in RNA expression following mono- and combination therapy. RNA-Seq revealed synergistic decreases in a number of genes including members of the JAK-STAT and MAPK pathways, which are druggable targets downstream of this transcriptional program. Further validation studies of these targets are currently underway and will be reported later.
The constitutive activation of NFAT has not been previously reported in KIT mutant cell lines. Further, our data suggest a point of convergence between KIT and NFAT signaling pathways, possibly through the interaction of NFAT with a DNA binding partner. Importantly, our results indicate that combination therapy using an NFAT inhibitor and a KIT kinase inhibitor results in synergistic killing of KIT mutant mast cells. However, existing calcineurin phosphatase inhibitors such as CSA have known chronic long-term toxicities, including immunosuppression. To identify alternative drug targets we used RNA-Seq and found that simultaneous calcineurin and KIT inhibition modulated members of the JAK-STAT and MAPK pathways. Preliminary data indicates that targeting these pathways in combination with dasatinib achieves the same synergistic effect on KIT mutant cancer cells. We are currently testing the potential of these alternative combination therapies for the treatment of advanced mast cell disease.
Heinrich: Onyx: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Patents & Royalties, Research Funding; MolecularMD: Consultancy, Equity Ownership; AROG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.