Abstract
Signaling by the erythropoietin receptor (EpoR) is essential for the survival of definitive colony-forming unit-erythroid (CFU-e) progenitors and their erythroblast progeny. Here we used EpoR-null embryos to ask whether EpoR signaling might also exert essential non-survival functions in erythropoiesis.
To address this, we rescued EpoR-null fetal liver cells from death by transducing them in vitro with either the anti-apoptotic protein Bcl-xL, or, as control, with the wild-type EpoR. The Bcl-xL-transduced EpoR-null cells survived, expressed hemoglobin and underwent morphological erythroid maturation and enucleation. However, unlike exogenous EpoR, exogenous Bcl-xL was unable to support the formation of EpoR-null CFU-e colonies in methylcellulose. The absence of colonies was explained by the finding that the Bcl-xL-transduced progenitors underwent fewer cell divisions than equivalent EpoR-transduced cells (1.1 vs. 2.9 in 24 hr, respectively) and had a slower rate of intra-S phase DNA synthesis, suggesting longer S phase duration. Multispectral imaging showed that the Bcl-xL-transduced cells matured prematurely, attaining smaller cell and nuclear size and a lower nuclear/cytoplasmic ratio at earlier time points than EpoR-transduced cells. Premature maturation was also evident by flow cytometric analysis. Thus, EpoR-null fetal liver cells in vivo arrest in their differentiation at the transition from subset S0 (Ter119-neg CD71-low) to S1 (Ter119-neg CD71-high) (Pop et al, PLoS Biology 2010). Rescue with EpoR in vitro allows EpoR-null progenitors to resume differentiation, sequentially upregulating CD71 and Ter119. By contrast, rescue of EpoR-null cells with Bcl-xL results in their premature upregulation of Ter119 and failure to upregulate CD71 to high levels. The cell cycle and differentiation deficits in Bcl-xL-supported, EpoR-null erythropoiesis were associated with a slower loss of DNA methylation from the erythroid genome, and with slower erythroid gene transcription.
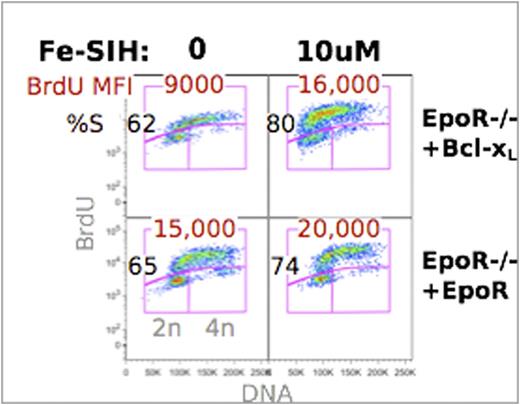
CD71 (the transferrin receptor) is a known target of EpoR and Stat5 signaling. We asked whether the deficits of EpoR-null erythropoiesis might be the result of low cell surface CD71 and the consequent reduced iron transport. In support of this hypothesis, we found that EpoR-null fetal liver cells that are transduced with both CD71 and Bcl-xL resume the normal maturation rate characteristic of EpoR-supported differentiation, as judged by multispectral imaging measurements of cell size and nuclear/cytoplasmic ratio. Further, we were able to restore rapid S phase to Bcl-xL-transduced EpoR-/- erythroblasts by culturing them in the presence of the cell-permeant iron chelator Fe-SIH (salicylaldehyde isonicotinoyl hydrazone), which is able to supply the cell interior with iron even in the absence of CD71 (Figure 1). We suggest that EpoR-mediated upregulation of CD71 at the onset of erythroid terminal differentiation determines the number and duration of erythroblast cell divisions by regulating iron homeostasis.
The cell-permeant iron chelator Fe-SIH rescues cycling in EpoR-/- fetal liver. EpoR-/- cells were transduced with EpoR or Bcl-xL and cultured in vitro in the presence or absence of 10uM Fe-SIH. Cells were pulsed with BrdU at 38hrs.
The cell-permeant iron chelator Fe-SIH rescues cycling in EpoR-/- fetal liver. EpoR-/- cells were transduced with EpoR or Bcl-xL and cultured in vitro in the presence or absence of 10uM Fe-SIH. Cells were pulsed with BrdU at 38hrs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.