Allogeneic hematopoietic stem cell transplantation is used to treat a number of acquired and congenital diseases; however, a major complication of this therapy is graft versus host disease (GvHD). GvHD is a severe inflammatory disease caused by donor T cells recognizing host organs as foreign and mounting an immune response against them. Current treatment for GvHD is an immunosuppressive drug regimen that can predispose patients to opportunistic infections. Therefore, it is of critical importance to further understand the underlying mechanisms that mediate GvHD pathogenesis in efforts to develop novel therapeutic treatments.
Previous work from our lab demonstrated that TH17 cells mediate severe cutaneous GvHD and that inhibiting IL-17 activity using a neutralizing anti-IL17 antibody was protective in a mouse model of skin GvHD. TH17 cells also produce the cytokine IL-22, which is known to be involved in skin inflammation resulting from psoriasis. We tested whether IL-22 from TH17 cells is also pathogenic in a mouse model of GvHD in which ex vivo polarized TH17 cells from B6 mice are transferred to lethally irradiated allogeneic B6D2 mice.
B6D2 recipient mice were lethally irradiated with 900 cGy from a Cesium source. The following day recipient mice were given 3 x 106 T cell depleted bone marrow cells supplemented with 3-4 x 106 Th17 cells that had been polarized in vitro from fluorescence activated cell sorted CD4+/CD62Lhi T cells that had been cultured as described previously (Carlson et al Blood 2009). Recipient mice were followed for survival and scored for clinical GVHD using a standard scoring system. Additionally, recipient mice were evaluated clinically for cutaneous GVHD and scored for tissue pathology. GVHD organs were evaluated for the production of pro-inflammatory cytokines by ELISA and qPCR. Treated animals received control IgG or anti-IL-22 antibody (16mg/kg) weekly for four weeks starting on the day of transplant (Day 0).
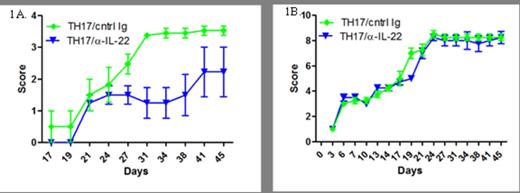
We found substantial expression of IL-22 mRNA in the skin of recipient mice who were transplanted with donor Th17 cells compared to those receiving naïve T cells alone (p< 0.05). When we evaluated the function of IL-22 produced by Th17 cells, we found a statistically significant decrease in cutaneous GVHD induced by Th17 cells in recipient animals treated with anti-IL-22 mAb (p< 0.05). While there was a decrease in cutaneous GVHD in the mice treated with anti-IL-22 mAb, there was no difference in overall survival or induction of GVHD outside of the cutaneous compartment. Additionally, we found increased expression of mRNA for IL-22 from a cohort of patients with late acute GVHD after allogeneic stem cell transplantation.
Unlike in the GI tract, the generation of IL-22 appears to play a pro-inflammatory role in the manifestation of cutaneous GVHD in mice after allogeneic bone marrow transplantation. High levels of mRNA for IL-22 were also found in the skin from a cohort of patients with late acute GVHD. These data suggest that targeting IL-22 may be beneficial in the treatment of cutaneous GVHD.
A.Cutaneous GvHD scoring. Mice transplanted with TH17 cells were treated with either 16mg/kg of control IgG or anti-IL-22 antibody once a week for four weeks. Mice were scored twice a week using an established scoring system: 0: No ulcers or alopecia; 1: Skin ulcers with alopecia less than 1cm2 in area; 2: 1-2 cm2 in area; 3: Greater than 2cm2 in area; an additional score of 0.3 is added if mice have ulcers or scaling on paws, tail, or ears. B. Acute GvHD scoring. Mice were scored twice a week using an established scoring system.
A.Cutaneous GvHD scoring. Mice transplanted with TH17 cells were treated with either 16mg/kg of control IgG or anti-IL-22 antibody once a week for four weeks. Mice were scored twice a week using an established scoring system: 0: No ulcers or alopecia; 1: Skin ulcers with alopecia less than 1cm2 in area; 2: 1-2 cm2 in area; 3: Greater than 2cm2 in area; an additional score of 0.3 is added if mice have ulcers or scaling on paws, tail, or ears. B. Acute GvHD scoring. Mice were scored twice a week using an established scoring system.
Fouser:Pfizer: Employment.