Allogeneic NK cells are anti-leukemia immune effector lymphocytes, with evidence of activity in patients in adoptive transfer clinical studies. What is the optimal approach to prepare allogeneic NK cells for maximal effector function remains an open question for adoptive NK cell therapy. Recently described cytokine-induced memory-like (CIML) NK cells are generated following a brief (16 hour) pre-activation with a combination of IL-12+IL-15+IL-18 in both mice and humans. Following weeks or months of rest, CIML NK cells exhibit an enhanced recall IFN-g response when restimulated with K562 cells or cytokines. However, their anti-leukemic cytotoxic activity and identification of key supporting cytokines for survival and sustained functionality have not been reported. We hypothesized that CIML NK cells may have enhanced effector function against AML, providing a potential rationale for future clinical studies of CIML NK cells in AML patients. To test this hypothesis we investigated the CIML NK cell response to myeloid leukemia, including primary AML blasts, and evaluated their function following transfer into NSG mice.
Normal human donor NK cells (>95% purity) were cultured with low dose (1 ng/mL) IL-15 alone (control) or pre-activated with IL-12 (10 ng/ml) + IL-15 (1 ng/ml) + IL-18 (50 ng/ml) for 16 hours. After washing, the cells were cultured for 7 days in low dose IL-15 (to support survival). Following this prolonged rest period in vitro, NK cell responses were assessed after 6-hour re-stimulation with K562 leukemia cells or primary AML blasts. NK cell functional responses assessed include IFN-g production and cytotoxicity (using flow based killing assays). For adoptive transfer experiments, 5-8 x 106 human CIML NK cells or control cells were injected into sub-lethally irradiated NSG mice, and assessed for persistence, expansion and function of the adoptively transferred CIML NK or control NK cell. Additional experiments included evaluating CIML NK cells for cytokine receptor expression and effector proteins after the 7 day rest period.
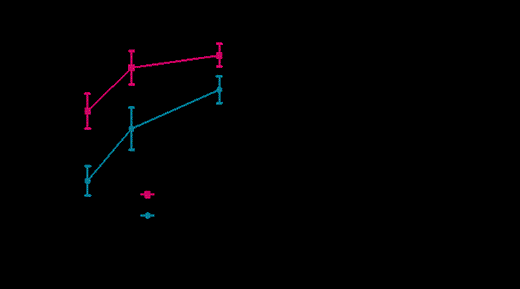
As described previously, CIML NK cells had a significantly increased IFN-g response to K562 leukemia cells (15.5 ± 3% vs. 7 ± 1%, P=0.03). CIML NK cells also exhibited a more potent IFN-g response to primary blasts from untreated, newly diagnosed AML patients (N=4 AML samples, P< 0.0001). Further, CIML NK cells demonstrated a significantly greater cytotoxic response, compared to control NK cells, upon co-incubation with K562 leukemia cells (Figure 1). Consistent with this enhanced cytotoxicity, CIML NK cells had significantly increased expression of granzyme A (P=0.005) and granzyme B (P=0.006) proteins. Further, we noted a marked induction of CD25 (IL-2Ra) after IL-12+IL-15+IL-18 pre-activation, which via the IL-2Rabg resulted in enhanced functional responses to picomolar concentrations of IL-2. This included enhanced cytotoxicity against leukemia cells, IFN-g production in response to co-stimulation with IL-12, and proliferation. To assess persistence and expansion upon adoptive transfer, NSG mice were injected with control or CIML NK cells. After 7 days (during which 75,000IU of IL-2 was injected qOD) there was a preferential expansion of the CIML NK cells in blood (11±2.6 vs. 5±1.3, P=0.01) and bone marrow (0.6±0.14 vs. 0.21±0.06, P= 0.03) in these mice as assessed by the ratio of human to mouse CD45 positive cells. Further, CIML NK cells supported in vivo in NSG mice exhibited enhanced IFN-g responses upon re-stimulation with K562 leukemia cells (10±1.5% vs. 2.5±1% IFN-g positive, P= 0.03) or IL-12+IL-15 (15±2% vs. 2±0.5%, P= 0.001).
Brief (16 hour) pre-activation with a combination of IL-12+IL-15+IL-18 results in the generation of CIML NK cells that have an enhanced IFN-g and cytotoxic response to K562 leukemia cells and primary allogeneic AML blasts. Further, CD25 is induced on CIML NK cells, which in the context of the high affinity IL-2Rabg confers selective responsiveness to low concentrations of IL-2 for proliferation, enhanced cytotoxicity, and enhanced IFN-g production. CIML NK cells may develop in vivo in NSG xenografts, and CIML NK cells appear to be selectively supported by exogenous low dose IL-2 in this context. These pre-clinical data support CIML NK cells as a novel optimization approach for NK cell adoptive immunotherapy.
No relevant conflicts of interest to declare.